当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical synthesis of 1,2,4-oxadiazoles from amidoximes through dehydrogenative cyclization
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-11-25 , DOI: 10.1039/d1ob02040d Chan Jiang 1 , Mingfang Li 1 , Leitao Xu 1 , Yangjie Yi 1 , Jiao Ye 1 , Aixi Hu 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-11-25 , DOI: 10.1039/d1ob02040d Chan Jiang 1 , Mingfang Li 1 , Leitao Xu 1 , Yangjie Yi 1 , Jiao Ye 1 , Aixi Hu 1
Affiliation
|
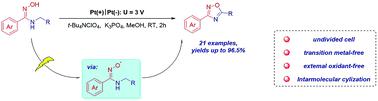
A convenient and efficient method for the generation of the iminoxy radical through anodic oxidation was developed for the synthesis of 3,5-disubstituted 1,2,4-oxadiazoles from N-benzyl amidoximes. The transformation proceeds through 1.5-Hydrogen Atom Transfer (1,5-HAT) and intramolecular cyclization. The process features simple operation, mild conditions, broad substrate scope and high functional group compatibility, and provides a facile and practical way for the preparation of 1,2,4-oxadiazoles.
中文翻译:

偕胺肟脱氢环化电化学合成1,2,4-恶二唑
为从N-苄基偕胺肟合成 3,5-二取代的 1,2,4-恶二唑,开发了一种通过阳极氧化生成亚胺氧基自由基的简便有效的方法。转化通过 1.5-氢原子转移 (1,5-HAT) 和分子内环化进行。该工艺操作简单、条件温和、底物范围广、官能团相容性高,为1,2,4-恶二唑的制备提供了一条简便实用的途径。
更新日期:2021-12-02
中文翻译:

偕胺肟脱氢环化电化学合成1,2,4-恶二唑
为从N-苄基偕胺肟合成 3,5-二取代的 1,2,4-恶二唑,开发了一种通过阳极氧化生成亚胺氧基自由基的简便有效的方法。转化通过 1.5-氢原子转移 (1,5-HAT) 和分子内环化进行。该工艺操作简单、条件温和、底物范围广、官能团相容性高,为1,2,4-恶二唑的制备提供了一条简便实用的途径。








































 京公网安备 11010802027423号
京公网安备 11010802027423号