Cell ( IF 45.5 ) Pub Date : 2021-12-02 , DOI: 10.1016/j.cell.2021.11.011 Liudmila Andreeva 1 , Liron David 1 , Shaun Rawson 2 , Chen Shen 1 , Teerithveen Pasricha 3 , Pablo Pelegrin 4 , Hao Wu 1
|
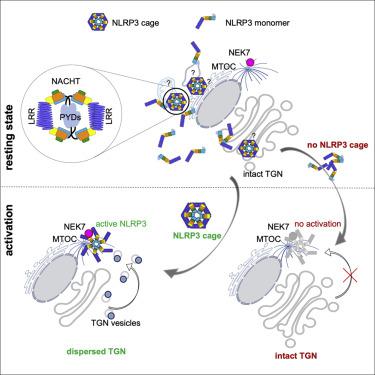
The NACHT-, leucine-rich-repeat- (LRR), and pyrin domain-containing protein 3 (NLRP3) is emerging to be a critical intracellular inflammasome sensor of membrane integrity and a highly important clinical target against chronic inflammation. Here, we report that an endogenous, stimulus-responsive form of full-length mouse NLRP3 is a 12- to 16-mer double-ring cage held together by LRR-LRR interactions with the pyrin domains shielded within the assembly to avoid premature activation. Surprisingly, this NLRP3 form is predominantly membrane localized, which is consistent with previously noted localization of NLRP3 at various membrane organelles. Structure-guided mutagenesis reveals that trans-Golgi network dispersion into vesicles, an early event observed for many NLRP3-activating stimuli, requires the double-ring cages of NLRP3. Double-ring-defective NLRP3 mutants abolish inflammasome punctum formation, caspase-1 processing, and cell death. Thus, our data uncover a physiological NLRP3 oligomer on the membrane that is poised to sense diverse signals to induce inflammasome activation.
中文翻译:

全长小鼠NLRP3结构控制通路激活揭示NLRP3笼
NACHT、富含亮氨酸重复序列 (LRR) 和含吡啶结构域的蛋白 3 (NLRP3) 正在成为膜完整性的关键细胞内炎症体传感器和对抗慢性炎症的非常重要的临床靶点。在这里,我们报告全长小鼠 NLRP3 的内源性刺激响应形式是一个 12 至 16 聚体双环笼,通过 LRR-LRR 与组装内屏蔽的热蛋白结构域相互作用保持在一起,以避免过早激活。令人惊讶的是,这种 NLRP3 形式主要是膜定位的,这与之前提到的 NLRP3 在各种膜细胞器中的定位一致。结构引导诱变揭示,反式高尔基体网络分散到囊泡中(许多 NLRP3 激活刺激物观察到的早期事件)需要 NLRP3 的双环笼。双环缺陷的 NLRP3 突变体消除了炎性小体泪点形成、caspase-1 加工和细胞死亡。因此,我们的数据揭示了膜上的生理性 NLRP3 寡聚体,它能够感知不同的信号以诱导炎症小体激活。































 京公网安备 11010802027423号
京公网安备 11010802027423号