European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-12-01 , DOI: 10.1016/j.ejmech.2021.114033 Yonghua Liu 1 , Jianrui Li 1 , Yuxi Gu 1 , Ling Ma 1 , Shan Cen 1 , Zonggen Peng 1 , Laixing Hu 1
|
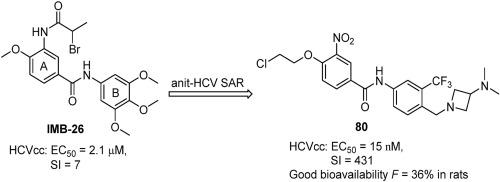
A series of novel biaryl amide derivatives were synthesized and evaluated for anti-HCV virus activity. Some significant SARs were uncovered. The intensive structural modifications led to fifteen novel compounds with more potent inhibitory activity compared to the hit compounds IMB 26 and IMB1f. Among them, compound 80 was the most active, with EC50 values almost equivalent to the clinical drug telaprevir (EC50 = 15 nM). Furthermore, it also had a good safety and in vitro and oral pharmacokinetic (oral bioavailability in rats: 34%) profile, suggesting a highly drug-like nature. Compound 80represents a more promising scaffold for anti-HCV virus activity for further study.
中文翻译:

新型联芳基酰胺衍生物的合成、构效关系研究及其对丙型肝炎病毒的抑制作用
合成了一系列新型联芳基酰胺衍生物,并评估了其抗 HCV 病毒活性。发现了一些重要的 SAR。与命中化合物IMB 26和IMB1f相比,密集的结构修饰产生了 15 种具有更强抑制活性的新型化合物。其中,化合物80的活性最高,EC 50值几乎与临床药物telaprevir相当(EC 50 = 15 nM)。此外,它还具有良好的安全性以及体外和口服药代动力学(大鼠口服生物利用度:34%)特征,表明具有高度类药性。化合物80代表了一种更有前途的抗 HCV 病毒活性支架,有待进一步研究。































 京公网安备 11010802027423号
京公网安备 11010802027423号