当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Artificial Biomolecular Channels: Enantioselective Transmembrane Transport of Amino Acids Mediated by Homochiral Zirconium Metal–Organic Cages
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-12-01 , DOI: 10.1021/jacs.1c09992 Yingguo Li 1 , Jinqiao Dong 1 , Wei Gong 1 , Xianhui Tang 1 , Yuhao Liu 1 , Yong Cui 1 , Yan Liu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-12-01 , DOI: 10.1021/jacs.1c09992 Yingguo Li 1 , Jinqiao Dong 1 , Wei Gong 1 , Xianhui Tang 1 , Yuhao Liu 1 , Yong Cui 1 , Yan Liu 1
Affiliation
|
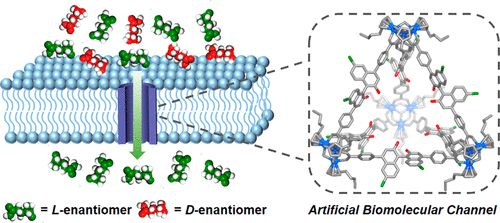
Natural transport channels (or carriers), such as aquaporins, are a distinct type of biomacromolecule capable of highly effective transmembrane transport of water or ions. Such behavior is routine for biology but has proved difficult to achieve in synthetic systems. Perhaps most significantly, the enantioselective transmembrane transport of biomolecules is an especially challenging problem both for chemists and for natural systems. Herein, a group of homochiral zirconium metal–organic cages with four triangular opening windows have been proposed as artificial biomolecular channels for enantioselective transmembrane transport of natural amino acids. These structurally well-defined coordination cages are assembled from six synthetically accessible BINOL-derived chiral ligands as spacers and four n-Bu3-Cp3Zr3 clusters as vertices, forming tetrahedral-shaped architectures that feature an intrinsically chiral cavity decorated with an array of specifically positioned binding sites mediated from phenol to phenyl ether to crown ether groups. Fascinatingly, the transformation of single-molecule chirality to global supramolecular chirality within the space-restricted chiral microenvironments accompanies unprecedented chiral amplification, leading to the enantiospecific recognition of amino acids. By virtue of the highly structural stability and excellent biocompatibility, the orientation-independent cages can be molecularly embedded into lipid membranes, biomimetically serving as single-molecular chiral channels for polar-residue amino acids, with the properties that cage-1 featuring hydroxyl groups preferentially transports the l-asparagine, whereas cage-2 attaching crown ether groups spontaneously favor transporting d-arginine. We therefore develop a new type of self-assembled system that can potentially mimic the functions of transmembrane proteins in nature, which is a realistic candidate for further biomedical applications.
中文翻译:

人工生物分子通道:由同手性锆金属-有机笼介导的氨基酸对映选择性跨膜转运
天然运输通道(或载体),例如水通道蛋白,是一种独特类型的生物大分子,能够高效地跨膜运输水或离子。这种行为对生物学来说是常规的,但在合成系统中很难实现。也许最重要的是,生物分子的对映选择性跨膜转运对于化学家和自然系统来说都是一个特别具有挑战性的问题。在此,一组具有四个三角形开口窗的纯手性锆金属-有机笼被提出作为人工生物分子通道,用于天然氨基酸的对映选择性跨膜转运。这些结构明确的配位笼由六个可合成的 BINOL 衍生的手性配体作为间隔物和四个n- Bu组装而成3 -Cp 3 Zr 3簇作为顶点,形成四面体形结构,其特征在于本质上手性的空腔装饰有一系列从苯酚到苯醚到冠醚基团介导的特定定位的结合位点。令人着迷的是,在空间受限的手性微环境中,单分子手性向全局超分子手性的转变伴随着前所未有的手性放大,导致氨基酸的对映体特异性识别。凭借高度的结构稳定性和优异的生物相容性,定向无关的笼子可以分子嵌入脂质膜中,仿生地充当极性残基氨基酸的单分子手性通道,具有笼子-具有羟基的1优先运输l-天冬酰胺,而连接冠醚基团的cage- 2自发地有利于运输d-精氨酸。因此,我们开发了一种新型的自组装系统,可以潜在地模仿自然界中跨膜蛋白的功能,这是进一步生物医学应用的现实候选者。
更新日期:2021-12-15
中文翻译:

人工生物分子通道:由同手性锆金属-有机笼介导的氨基酸对映选择性跨膜转运
天然运输通道(或载体),例如水通道蛋白,是一种独特类型的生物大分子,能够高效地跨膜运输水或离子。这种行为对生物学来说是常规的,但在合成系统中很难实现。也许最重要的是,生物分子的对映选择性跨膜转运对于化学家和自然系统来说都是一个特别具有挑战性的问题。在此,一组具有四个三角形开口窗的纯手性锆金属-有机笼被提出作为人工生物分子通道,用于天然氨基酸的对映选择性跨膜转运。这些结构明确的配位笼由六个可合成的 BINOL 衍生的手性配体作为间隔物和四个n- Bu组装而成3 -Cp 3 Zr 3簇作为顶点,形成四面体形结构,其特征在于本质上手性的空腔装饰有一系列从苯酚到苯醚到冠醚基团介导的特定定位的结合位点。令人着迷的是,在空间受限的手性微环境中,单分子手性向全局超分子手性的转变伴随着前所未有的手性放大,导致氨基酸的对映体特异性识别。凭借高度的结构稳定性和优异的生物相容性,定向无关的笼子可以分子嵌入脂质膜中,仿生地充当极性残基氨基酸的单分子手性通道,具有笼子-具有羟基的1优先运输l-天冬酰胺,而连接冠醚基团的cage- 2自发地有利于运输d-精氨酸。因此,我们开发了一种新型的自组装系统,可以潜在地模仿自然界中跨膜蛋白的功能,这是进一步生物医学应用的现实候选者。

































 京公网安备 11010802027423号
京公网安备 11010802027423号