当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multifunctional Electrolyte Additive Stabilizes Electrode–Electrolyte Interface Layers for High-Voltage Lithium Metal Batteries
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-11-29 , DOI: 10.1021/acsami.1c18783 Yongchao Liu 1, 2 , Liu Hong 1 , Rui Jiang 1 , Yueda Wang 1, 2 , Sawankumar V Patel 3 , Xuyong Feng 1, 2 , Hongfa Xiang 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-11-29 , DOI: 10.1021/acsami.1c18783 Yongchao Liu 1, 2 , Liu Hong 1 , Rui Jiang 1 , Yueda Wang 1, 2 , Sawankumar V Patel 3 , Xuyong Feng 1, 2 , Hongfa Xiang 1, 2
Affiliation
|
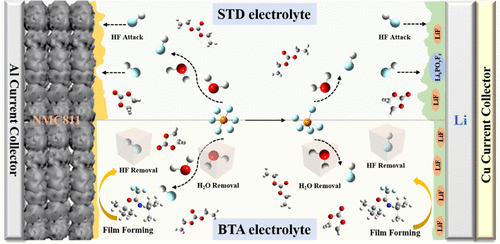
A lithium metal anode and high nickel ternary cathode are considered viable candidates for high energy density lithium metal batteries (LMBs). However, unstable electrode–electrolyte interfaces and structure degradation of high nickel ternary cathode materials lead to serious capacity decay, consequently hindering their practical applications in LMBs. Herein, we introduced N,O-bis(trimethylsilyl) trifluoro acetamide (BTA) as a multifunctional additive for removing trace water and hydrofluoric acid (HF) from the electrolyte and inhibiting corrosive HF from disrupting the electrode–electrolyte interface layers. Furthermore, the BTA additive containing multiple functional groups (C–F, Si–O, Si–N, and C═N) promotes the formation of LiF-rich, Si- and N-containing solid electrolyte interfacial films on a lithium metal anode and LiNi0.8Mn0.1Co0.1O2 (NMC811) cathode surfaces, thereby improving the electrode–electrolytes interfacial stability and mitigating the capacity decay caused by structural degradation of the layered cathode. Using the BTA additive had tremendous benefits through modification of both anode and cathode surface layers. This was demonstrated using a Li||NMC811 metal battery with the BTA electrolyte, which exhibits remarkable cycling and rate performances (122.9 mA h g–1 at 10 C) and delivers a discharge capacity of 162 mA h g–1 after 100 cycles at 45 °C. Likewise, this study establishes a cost-effective approach of using a single additive to improve the electrochemical performance of LMBs.
中文翻译:

多功能电解质添加剂可稳定高压锂金属电池的电极-电解质界面层
锂金属阳极和高镍三元阴极被认为是高能量密度锂金属电池 (LMB) 的可行候选者。然而,不稳定的电极-电解质界面和高镍三元正极材料的结构退化导致严重的容量衰减,从而阻碍了它们在LMBs中的实际应用。在这里,我们介绍了N , O-双(三甲基甲硅烷基)三氟乙酰胺(BTA)作为多功能添加剂,用于从电解质中去除痕量水和氢氟酸(HF),并抑制腐蚀性 HF 破坏电极-电解质界面层。此外,含有多个官能团(C-F、Si-O、Si-N 和 C=N)的 BTA 添加剂促进了在锂金属负极上形成富含 LiF、含 Si 和 N 的固体电解质界面膜和 LiNi 0.8 Mn 0.1 Co 0.1 O 2(NMC811) 阴极表面,从而提高电极-电解质界面稳定性并减轻由层状阴极结构退化引起的容量衰减。通过阳极和阴极表面层的改性,使用 BTA 添加剂具有巨大的好处。使用带有 BTA 电解质的 Li||NMC811 金属电池证明了这一点,该电池表现出显着的循环和倍率性能(10 C 下为122.9 mA hg –1),并在 45 ° 下循环 100 次后提供 162 mA hg –1的放电容量C。同样,这项研究建立了一种使用单一添加剂来提高 LMB 电化学性能的经济有效的方法。
更新日期:2021-12-08
中文翻译:

多功能电解质添加剂可稳定高压锂金属电池的电极-电解质界面层
锂金属阳极和高镍三元阴极被认为是高能量密度锂金属电池 (LMB) 的可行候选者。然而,不稳定的电极-电解质界面和高镍三元正极材料的结构退化导致严重的容量衰减,从而阻碍了它们在LMBs中的实际应用。在这里,我们介绍了N , O-双(三甲基甲硅烷基)三氟乙酰胺(BTA)作为多功能添加剂,用于从电解质中去除痕量水和氢氟酸(HF),并抑制腐蚀性 HF 破坏电极-电解质界面层。此外,含有多个官能团(C-F、Si-O、Si-N 和 C=N)的 BTA 添加剂促进了在锂金属负极上形成富含 LiF、含 Si 和 N 的固体电解质界面膜和 LiNi 0.8 Mn 0.1 Co 0.1 O 2(NMC811) 阴极表面,从而提高电极-电解质界面稳定性并减轻由层状阴极结构退化引起的容量衰减。通过阳极和阴极表面层的改性,使用 BTA 添加剂具有巨大的好处。使用带有 BTA 电解质的 Li||NMC811 金属电池证明了这一点,该电池表现出显着的循环和倍率性能(10 C 下为122.9 mA hg –1),并在 45 ° 下循环 100 次后提供 162 mA hg –1的放电容量C。同样,这项研究建立了一种使用单一添加剂来提高 LMB 电化学性能的经济有效的方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号