Molecular Therapy: Oncology ( IF 5.3 ) Pub Date : 2021-11-29 , DOI: 10.1016/j.omto.2021.11.020 Rūta Veinalde 1 , Gemma Pidelaserra-Martí 1, 2, 3 , Coline Moulin 2, 4 , Lara M Jeworowski 1 , Linda Küther 2 , Christian J Buchholz 5 , Dirk Jäger 6, 7 , Guy Ungerechts 1, 6 , Christine E Engeland 1, 2, 6
|
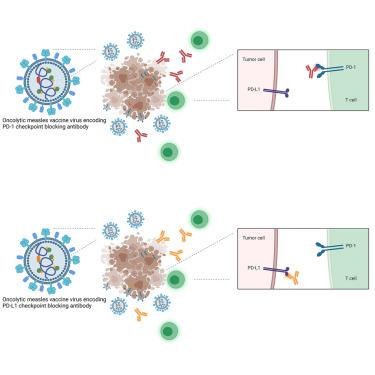
PD-1/PD-L1 checkpoint blockade has achieved unprecedented success in cancer immunotherapy. Nevertheless, many immune-excluded tumors are resistant to therapy. Combination with oncolytic virotherapy may overcome resistance by inducing acute inflammation, immune cell recruitment, and remodeling of the tumor immune environment. Here, we assessed the combination of oncolytic measles vaccine (MV) vectors and PD-1/PD-L1 blockade. In the MC38cea model of measles virus oncolysis, MV combined with anti-PD-1 and MV vectors encoding anti-PD-1 or anti-PD-L1 antibodies achieved modest survival benefits compared with control MV or vectors encoding the antibody constant regions only. Analyses of tumor samples and tumor-draining lymph nodes revealed slight increases in intratumoral T cell effector cytokines as well as a shift toward an effector memory phenotype in the T cell compartment. Importantly, increased IFN-γ recall responses were observed in tumor rechallenge experiments with mice in complete tumor remission after treatment with MV encoding anti-PD-1 or anti-PD-L1 compared with control MV. These results prompted us to generate MV encoding the clinically approved agents pembrolizumab and nivolumab. Previously, we have generated MV encoding atezolizumab. We demonstrated the functionality of the novel vectors in vitro. We envision these vectors as therapeutics that induce and support durable anti-tumor immune memory.
中文翻译:

编码 PD-1 和 PD-L1 检查点阻断抗体的溶瘤麻疹疫苗可增加肿瘤特异性 T 细胞记忆
PD-1/PD-L1 检查点阻断在癌症免疫治疗中取得了前所未有的成功。然而,许多免疫排斥肿瘤对治疗具有抗性。与溶瘤病毒疗法相结合,可以通过诱导急性炎症、免疫细胞募集和肿瘤免疫环境的重塑来克服耐药性。在这里,我们评估了溶瘤麻疹疫苗 (MV) 载体和 PD-1/PD-L1 阻断剂的组合。在麻疹病毒溶瘤的 MC38cea 模型中,与对照 MV 或仅编码抗体恒定区的载体相比,MV 与抗 PD-1 和编码抗 PD-1 或抗 PD-L1 抗体的 MV 载体结合实现了适度的生存获益。对肿瘤样本和肿瘤引流淋巴结的分析显示,肿瘤内 T 细胞效应细胞因子略有增加,并且 T 细胞区室中向效应记忆表型转变。重要的是,与对照 MV 相比,在用编码抗 PD-1 或抗 PD-L1 的 MV 治疗后肿瘤完全缓解的小鼠的肿瘤再激发实验中观察到增加的 IFN-γ 回忆反应。这些结果促使我们生成编码临床批准的药物 pembrolizumab 和 nivolumab 的 MV。以前,我们已经生成了编码 atezolizumab 的 MV。我们展示了新载体的功能 与对照 MV 相比,在用编码抗 PD-1 或抗 PD-L1 的 MV 治疗后肿瘤完全缓解的小鼠的肿瘤再激发实验中观察到 IFN-γ 回忆反应增加。这些结果促使我们生成编码临床批准的药物 pembrolizumab 和 nivolumab 的 MV。以前,我们已经生成了编码 atezolizumab 的 MV。我们展示了新载体的功能 与对照 MV 相比,在用编码抗 PD-1 或抗 PD-L1 的 MV 治疗后肿瘤完全缓解的小鼠的肿瘤再激发实验中观察到 IFN-γ 回忆反应增加。这些结果促使我们生成编码临床批准的药物 pembrolizumab 和 nivolumab 的 MV。以前,我们已经生成了编码 atezolizumab 的 MV。我们展示了新载体的功能体外。我们将这些载体设想为诱导和支持持久抗肿瘤免疫记忆的疗法。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号