Applied Energy ( IF 10.1 ) Pub Date : 2021-11-27 , DOI: 10.1016/j.apenergy.2021.118158 Yu Luo 1 , Shuting Liao 1 , Shuai Chen 1 , Huihuang Fang 1 , Fulan Zhong 1 , Li Lin 1 , Chen Zhou 1 , Chongqi Chen 1 , Guohui Cai 1 , Chak-Tong Au 1 , Lilong Jiang 1
|
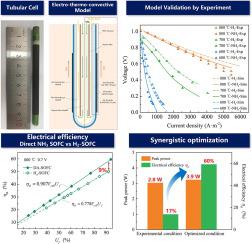
With high energy density both by weight and volume, ammonia (NH3) is a promising hydrogen carrier. Furthemore, NH3 has a mature industrial background, and in liquid form storage and transportation is not a problem. Adding the merit of zero CO2 emission, NH3-to-power by direct ammonia solid oxide fuel cells (DA-SOFCs) is an acceptable strategy to facilitate hydrogen usage. Nonetheless, to achieve efficacy, a high compatibility between operating temperature and catalytic materials for NH3 decomposition is needed. In this work, we developed a tubular DA-SOFC with an output power capability of > 3 W. By combining experimental measurements and multi-physics simulation, we comprehensively studies the related intrinsic processes. Based on experimental data, we developed a two-dimensional multi-scale electro-thermo model of tubular DA-SOFC. Separately we evaluated the effects of inlet fuel gas composition, inlet flow velocity, operating temperature, and operating voltage on the rate of NH3 catalytic decomposition and H2 electrochemical oxidation, as well as on NH3 conversion, H atom utilization, and electrical efficiency of the tubular DA-SOFC. The results suggest that high H atom utilization could be realized by matching the rate of NH3 decomposition with that of H2 electrochemical oxidation. It was observed that with the decrease of temperature, the rate of H2 oxidation decreases more rapidly than that of NH3 decomposition, suggesting that the flow velocity of NH3 should be appropriately lowered to optimize H atom utilization. Finally, we established a correlation between H atom utilization, operating voltage, and electrical efficiency for synergistic optimization of operating conditions. At 0.7 V and 800 ℃, the tubular DA-SOFC fueled with NH3 of 27 mL·min−1 is capable of offering 3.2 W, displaying an efficiency of 60%. Compared to that of a tubular H2-SOFC (only 51% efficiency), the efficiency is significantly higher on the basis of equal voltage and fuel utilization ratio. The outcome of the present study demonstrates the potential of tubular DA-SOFC as a device for high-efficiency power generation.
中文翻译:

管式直接氨固体氧化物燃料电池中氨分解和电化学氧化的优化耦合用于高效发电
氨(NH 3)具有高的重量和体积能量密度,是一种很有前途的氢载体。此外,NH 3具有成熟的工业背景,液态储存和运输不成问题。通过直接氨固体氧化物燃料电池 (DA-SOFC)添加零 CO 2排放、NH 3到电力的优点是促进氢使用的可接受策略。尽管如此,为了实现功效,操作温度和 NH 3催化材料之间的高度兼容性需要分解。在这项工作中,我们开发了一种输出功率能力 > 3 W 的管状 DA-SOFC。通过结合实验测量和多物理场模拟,我们全面研究了相关的内在过程。基于实验数据,我们开发了管状 DA-SOFC 的二维多尺度电热模型。我们分别评估了入口燃气组成、入口流速、工作温度和工作电压对 NH 3催化分解和 H 2电化学氧化速率的影响,以及对 NH 3转化率、H 原子利用率和电效率的影响管状 DA-SOFC。结果表明,通过匹配 NH 的速率可以实现高 H 原子利用率。3分解与H 2电化学氧化。观察到随着温度的降低,H 2氧化的速度比NH 3分解的速度降低得更快,这表明应该适当降低NH 3的流速以优化H原子的利用。最后,我们建立了 H 原子利用率、工作电压和电效率之间的相关性,以协同优化工作条件。在0.7 V和800 ℃下,以27 mL·min -1 NH 3为燃料的管状DA-SOFC能够提供3.2 W,显示效率为60%。与管状 H 2 相比-SOFC(效率只有51%),在同等电压和燃料利用率的基础上,效率明显更高。本研究的结果证明了管状 DA-SOFC 作为高效发电设备的潜力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号