当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Dihydride and Dihydrogen Complexes in Hydrogen Evolution Reaction on Single-Atom Catalysts
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-25 , DOI: 10.1021/jacs.1c10470
Giovanni Di Liberto 1 , Luis A Cipriano 1 , Gianfranco Pacchioni 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-25 , DOI: 10.1021/jacs.1c10470
Giovanni Di Liberto 1 , Luis A Cipriano 1 , Gianfranco Pacchioni 1
Affiliation
|
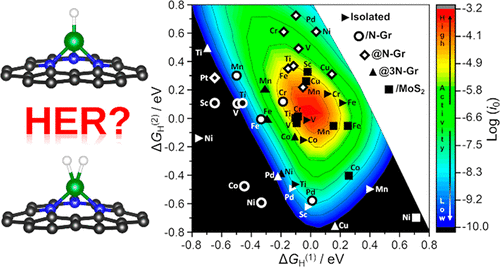
The hydrogen evolution reaction (HER) has a key role in electrochemical water splitting. Recently a lot of attention has been dedicated to HER from single atom catalysts (SACs). The activity of SACs in HER is usually rationalized or predicted using the original model proposed by Nørskov where the free energy of a H atom adsorbed on an extended metal surface M (formation of an MH intermediate) is used to explain the trends in the exchange current for HER. However, SACs differ substantially from metal surfaces and can be considered analogues of coordination compounds. In coordination chemistry, at variance with metal surfaces, stable dihydride or dihydrogen complexes (HMH) can form. We show that the same can occur on SACs and that the formation of stable HMH intermediates, in addition to the MH one, may change the kinetics of the process. Extending the original kinetic model to the case of two intermediates (MH and HMH), one obtains a three-dimensional volcano plot for the HER on SACs. DFT numerical simulations on 55 models demonstrate that the new kinetic model may lead to completely different conclusions about the activity of SACs in HER. The results are validated against selected experimental cases. The work provides an example of the important analogies between the chemistry of SACs and that of coordination compounds.
中文翻译:

二氢化物和二氢配合物在单原子催化剂析氢反应中的作用
析氢反应(HER)在电化学水分解中起着关键作用。最近,单原子催化剂(SAC)的 HER 受到了很多关注。 HER 中 SAC 的活性通常使用 Nørskov 提出的原始模型进行合理化或预测,其中吸附在扩展金属表面 M 上的 H 原子的自由能(形成 MH 中间体)用于解释交换电流的趋势为了她。然而,SAC 与金属表面有很大不同,可以被视为配位化合物的类似物。在配位化学中,与金属表面不同,可以形成稳定的二氢化物或二氢络合物(HMH)。我们表明,SAC 上也会发生同样的情况,并且除了 MH 之外,稳定的 HMH 中间体的形成可能会改变该过程的动力学。将原始动力学模型扩展到两种中间体(MH 和 HMH)的情况,可以获得 SAC 上 HER 的三维火山图。对 55 个模型的 DFT 数值模拟表明,新的动力学模型可能会得出关于 HER 中 SAC 活性的完全不同的结论。结果根据选定的实验案例进行了验证。这项工作提供了 SAC 化学与配位化合物化学之间重要相似性的一个例子。
更新日期:2021-12-08
中文翻译:

二氢化物和二氢配合物在单原子催化剂析氢反应中的作用
析氢反应(HER)在电化学水分解中起着关键作用。最近,单原子催化剂(SAC)的 HER 受到了很多关注。 HER 中 SAC 的活性通常使用 Nørskov 提出的原始模型进行合理化或预测,其中吸附在扩展金属表面 M 上的 H 原子的自由能(形成 MH 中间体)用于解释交换电流的趋势为了她。然而,SAC 与金属表面有很大不同,可以被视为配位化合物的类似物。在配位化学中,与金属表面不同,可以形成稳定的二氢化物或二氢络合物(HMH)。我们表明,SAC 上也会发生同样的情况,并且除了 MH 之外,稳定的 HMH 中间体的形成可能会改变该过程的动力学。将原始动力学模型扩展到两种中间体(MH 和 HMH)的情况,可以获得 SAC 上 HER 的三维火山图。对 55 个模型的 DFT 数值模拟表明,新的动力学模型可能会得出关于 HER 中 SAC 活性的完全不同的结论。结果根据选定的实验案例进行了验证。这项工作提供了 SAC 化学与配位化合物化学之间重要相似性的一个例子。































 京公网安备 11010802027423号
京公网安备 11010802027423号