Separation and Purification Technology ( IF 8.1 ) Pub Date : 2021-11-25 , DOI: 10.1016/j.seppur.2021.120199 Yuxuan He 1, 2 , Jin Qian 1, 2 , Bin Xu 3 , Peifang Wang 1, 2 , Bianhe Lu 1, 2 , Sijing Tang 1, 2 , Pan Gao 1, 2
|
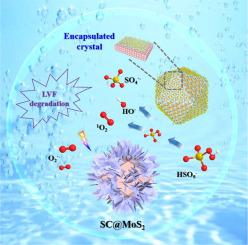
Perovskite oxides and molybdenum sulfide (MoS2) are both promising catalysts for advanced oxidation processes in environmental pollutant degradation. Herein, a core-shell structure of MoS2 encapsulating Sr-Co perovskite microcrystalline (SC@MoS2) was used to activate peroxymonosulfate (PMS) for degradation of organics. Levofloxacin (LVF) degradation by SC@MoS2/PMS system was improved obviously, and 0.2-SC@MoS2 exhibited the optimized activation for PMS with best LVF degradation performance (97%) within 15 min. In the 0.2-SC@MoS2/PMS system, the dosage of catalyst was the key determinant of LVF degradation. Simultaneously, 0.2-SC@MoS2 achieved outstanding reusability and good degradation capacity of different contaminants. The encapsulation of molybdenum disulfide layer facilitated electron transfer between PMS and core-shell structure. According to the EPR analysis and quenching experiments, both radicals (SO4•- and •OH), and nonradical (1O2) were involved in the degradation of LVF. Under visible light irradiation, O2•- participated in the reaction and enhanced LVF degradation efficiency, because the valence band and conduction band of components are different. Furthermore, the density functional theory (DFT) calculation, intermediates determination and Toxicity Estimation Software Tool (T.E.S.T) analysis provided meaningful support in the degradation pathways and ecological risks of LVF residues.
中文翻译:

将 SrCoO3 钙钛矿晶体封装在二硫化钼层内作为核壳结构,以增强过硫酸盐活化的电子转移
钙钛矿氧化物和硫化钼 (MoS 2 ) 都是用于环境污染物降解的高级氧化过程的有前途的催化剂。在此,MoS 2包覆Sr-Co钙钛矿微晶(SC@MoS 2)的核壳结构用于活化过硫酸盐(PMS)以降解有机物。左氧氟沙星(LVF)由SC @降解的MoS 2 / PMS系统明显提高,并且0.2-SC@MoS 2表现出优化的活化与PMS 15分钟内最好LVF降解性能(97%)。在0.2-SC@MoS 2 /PMS体系中,催化剂用量是LVF降解的关键决定因素。同时,0.2-SC@MoS 2实现了出色的重复使用性和对不同污染物的良好降解能力。二硫化钼层的封装促进了 PMS 和核壳结构之间的电子转移。根据 EPR 分析和淬灭实验,自由基(SO 4 •-和• OH)和非自由基(1 O 2)都参与了 LVF 的降解。在可见光照射下,O 2 •-参与反应并增强了 LVF 降解效率,因为组分的价带和导带不同。此外,密度泛函理论 (DFT) 计算、中间体测定和毒性估计软件工具 (TEST) 分析为 LVF 残留物的降解途径和生态风险提供了有意义的支持。






























 京公网安备 11010802027423号
京公网安备 11010802027423号