当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deep Sequencing of a Systematic Peptide Library Reveals Conformationally-Constrained Protein Interface Peptides that Disrupt a Protein-Protein Interaction
ChemBioChem ( IF 2.6 ) Pub Date : 2021-11-24 , DOI: 10.1002/cbic.202100504 David M Boragine 1 , Wanzhi Huang 2 , Lynn H Su 2 , Timothy Palzkill 2
ChemBioChem ( IF 2.6 ) Pub Date : 2021-11-24 , DOI: 10.1002/cbic.202100504 David M Boragine 1 , Wanzhi Huang 2 , Lynn H Su 2 , Timothy Palzkill 2
Affiliation
|
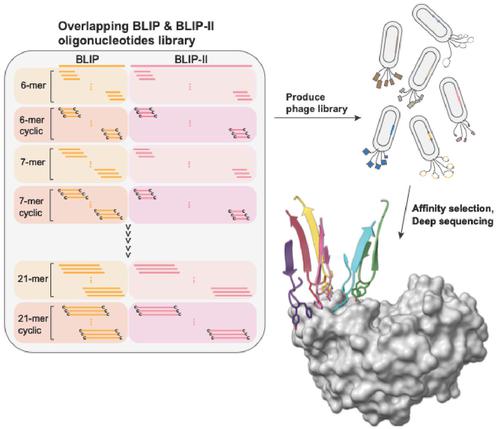
Disrupting protein-protein interactions is difficult due to the large and flat interaction surfaces of the binding partners. We utilized high throughput oligonucleotide synthesis, peptide phage display library construction, affinity selection, and deep sequencing to show a peptide library encompassing a binding partner in a protein-protein interaction can serve as a source of peptides that mimic the interface and provide a starting point for developing inhibitors that block the interface.

中文翻译:

系统肽库的深度测序揭示了破坏蛋白质-蛋白质相互作用的构象受限蛋白质界面肽
由于结合配偶体的相互作用表面又大又平,破坏蛋白质-蛋白质相互作用很困难。我们利用高通量寡核苷酸合成、肽噬菌体展示库构建、亲和力选择和深度测序来证明包含蛋白质-蛋白质相互作用中的结合配偶体的肽库可以作为模拟界面的肽的来源并提供起点用于开发阻断界面的抑制剂。

更新日期:2021-11-24

中文翻译:

系统肽库的深度测序揭示了破坏蛋白质-蛋白质相互作用的构象受限蛋白质界面肽
由于结合配偶体的相互作用表面又大又平,破坏蛋白质-蛋白质相互作用很困难。我们利用高通量寡核苷酸合成、肽噬菌体展示库构建、亲和力选择和深度测序来证明包含蛋白质-蛋白质相互作用中的结合配偶体的肽库可以作为模拟界面的肽的来源并提供起点用于开发阻断界面的抑制剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号