Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2021-11-24 , DOI: 10.1016/j.bmcl.2021.128479 Aaron M Bender 1 , Trever R Carter 1 , Matthew Spock 1 , Alice L Rodriguez 1 , Jonathan W Dickerson 1 , Jerri M Rook 1 , Sichen Chang 1 , Aidong Qi 1 , Christopher C Presley 1 , Darren W Engers 1 , Joel M Harp 2 , Thomas M Bridges 1 , Colleen M Niswender 3 , P Jeffrey Conn 3 , Craig W Lindsley 4
|
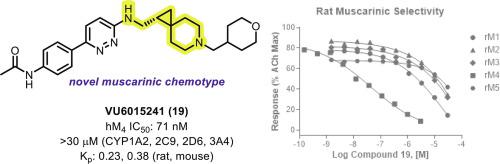
In this manuscript, we report a series of chiral 6-azaspiro[2.5]octanes and related spirocycles as highly potent and selective antagonists of the muscarinic acetylcholine receptor subtype 4 (mAChR4). Chiral separation and subsequent X-ray crystallographic analysis of early generation analogs revealed the R enantiomer to possess excellent human and rat M4 potency, and further structure–activity relationship (SAR) studies on this chiral scaffold led to the discovery of VU6015241 (compound 19). Compound 19 is characterized by high M4 potency and selectivity across multiple species, excellent aqueous solubility, and moderate brain exposure in rodents after intraperitoneal administration.
中文翻译:

手性 6-氮杂螺[2.5]辛烷作为 M4 毒蕈碱乙酰胆碱受体的有效和选择性拮抗剂的合成和表征
在这份手稿中,我们报告了一系列手性 6-氮杂螺[2.5]辛烷和相关螺环作为毒蕈碱乙酰胆碱受体亚型 4 (mAChR 4 )的高效和选择性拮抗剂。早期类似物的手性分离和随后的 X 射线晶体学分析表明,R对映异构体具有出色的人类和大鼠 M 4效力,并且对该手性支架的进一步构效关系 (SAR) 研究导致发现了 VU6015241(化合物19)。化合物19的特点是高 M 4对多个物种的效力和选择性,优异的水溶性,以及腹腔内给药后啮齿动物的适度脑暴露。






























 京公网安备 11010802027423号
京公网安备 11010802027423号