Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2021-11-25 , DOI: 10.1016/j.omtm.2021.11.011 Marti Cabanes-Creus 1 , Renina Gale Navarro 1 , Erhua Zhu 2 , Grober Baltazar 1 , Sophia H Y Liao 1 , Matthieu Drouyer 1 , Anais K Amaya 2 , Suzanne Scott 2, 3 , Loan Hanh Nguyen 1 , Adrian Westhaus 1, 4 , Matthias Hebben 5 , Laurence O W Wilson 3 , Adrian J Thrasher 4 , Ian E Alexander 2, 6 , Leszek Lisowski 1, 7, 8
|
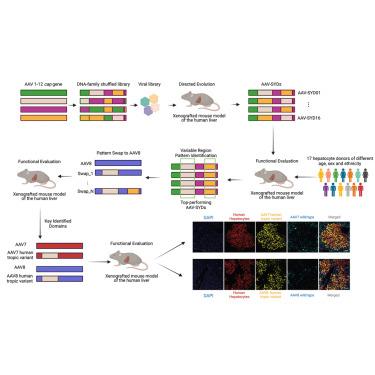
Recent clinical successes have intensified interest in using adeno-associated virus (AAV) vectors for therapeutic gene delivery. The liver is a key clinical target, given its critical physiological functions and involvement in a wide range of genetic diseases. Here, we report the bioengineering of a set of next-generation AAV vectors, named AAV-SYDs (where “SYD” stands for Sydney, Australia), with increased human hepato-tropism in a liver xenograft mouse model repopulated with primary human hepatocytes. We followed a two-step process that staggered directed evolution and domain-swapping approaches. Using DNA-family shuffling, we first mapped key AAV capsid regions responsible for efficient human hepatocyte transduction in vivo. Focusing on these regions, we next applied domain-swapping strategies to identify and study key capsid residues that enhance primary human hepatocyte uptake and transgene expression. Our findings underscore the potential of AAV-SYDs as liver gene therapy vectors and provide insights into the mechanism responsible for their enhanced transduction profile.
中文翻译:

新型人类肝向性 AAV 变体定义了可显着增强 AAV7 和 AAV8 人类向性的可转移结构域
最近的临床成功增强了人们对使用腺相关病毒 (AAV) 载体进行治疗性基因传递的兴趣。鉴于肝脏具有重要的生理功能并与多种遗传疾病有关,因此肝脏是一个关键的临床靶点。在这里,我们报告了一组下一代 AAV 载体的生物工程,称为 AAV-SYD(其中“SYD”代表澳大利亚悉尼),在重新填充原代人肝细胞的肝脏异种移植小鼠模型中具有增强的人肝向性。我们遵循了一个两步过程,交错了定向进化和域交换方法。利用 DNA 家族改组,我们首先绘制了负责体内高效人肝细胞转导的关键 AAV 衣壳区域。着眼于这些区域,我们接下来应用域交换策略来识别和研究增强原代人肝细胞摄取和转基因表达的关键衣壳残基。我们的研究结果强调了 AAV-SYD 作为肝脏基因治疗载体的潜力,并提供了对其增强转导谱的机制的见解。





























 京公网安备 11010802027423号
京公网安备 11010802027423号