当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Protein Hydration Dynamics and Molecular Mechanism of Coupled Water−Protein Fluctuations
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-08-05 , DOI: 10.1021/ja902918p Luyuan Zhang 1 , Yi Yang 1 , Ya-Ting Kao 1 , Lijuan Wang 1 , Dongping Zhong 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-08-05 , DOI: 10.1021/ja902918p Luyuan Zhang 1 , Yi Yang 1 , Ya-Ting Kao 1 , Lijuan Wang 1 , Dongping Zhong 1
Affiliation
|
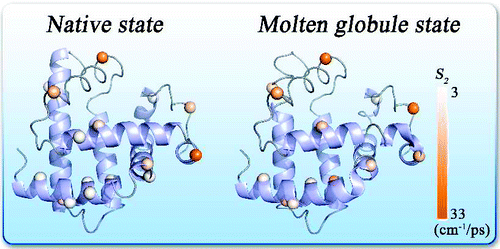
Protein surface hydration is fundamental to its structural stability and flexibility, and water-protein fluctuations are essential to biological function. Here, we report a systematic global mapping of water motions in the hydration layer around a model protein of apomyoglobin in both native and molten globule states. With site-directed mutagenesis, we use intrinsic tryptophan as a local optical probe to scan the protein surface one at a time with single-site specificity. With femtosecond resolution, we examined 16 mutants in two states and observed two types of water-network relaxation with distinct energy and time distributions. The first water motion results from the local collective hydrogen-bond network relaxation and occurs in a few picoseconds. The initial hindered motions, observed in bulk water in femtoseconds, are highly suppressed and drastically slow down due to structured water-network collectivity in the layer. The second water-network relaxation unambiguously results from the lateral cooperative rearrangements in the inner hydration shell and occurs in tens to hundreds of picoseconds. Significantly, this longtime dynamics is the coupled interfacial water-protein motions and is the direct measurement of such cooperative fluctuations. These local protein motions, although highly constrained, are necessary to assist the longtime water-network relaxation. A series of correlations of hydrating water dynamics and coupled fluctuations with local protein's chemical and structural properties were observed. These results are significant and reveal various water behaviors in the hydration layer with wide heterogeneity. We defined a solvation speed and an angular speed to quantify the water-network rigidity and local protein flexibility, respectively. We also observed that the dynamic hydration layer extends to more than 10 A. Finally, from native to molten globule states, the hydration water networks loosen up, and the protein locally becomes more flexible with larger global plasticity and partial unfolding.
中文翻译:

耦合水-蛋白质波动的蛋白质水合动力学和分子机制
蛋白质表面水化是其结构稳定性和灵活性的基础,水-蛋白质波动对生物功能至关重要。在这里,我们报告了在天然和熔融球状态下的脱脂蛋白肌红蛋白模型蛋白周围水化层中水运动的系统全局映射。通过定点诱变,我们使用内在色氨酸作为局部光学探针以单点特异性一次一个地扫描蛋白质表面。通过飞秒分辨率,我们检查了两种状态下的 16 个突变体,并观察到两种具有不同能量和时间分布的水网络弛豫。第一次水运动由局部集体氢键网络弛豫引起,发生在几皮秒内。在飞秒内在大量水中观察到的初始受阻运动,由于层中的结构化水网络集体性,受到高度抑制并显着减慢。第二次水网络松弛明确地由内部水化壳中的横向协同重排引起,发生在数十到数百皮秒内。重要的是,这种长期动力学是耦合的界面水-蛋白质运动,是这种协同波动的直接测量。这些局部蛋白质运动虽然受到高度限制,但对于帮助长时间的水网络松弛是必要的。观察到一系列水化水动力学和耦合波动与局部蛋白质的化学和结构特性的相关性。这些结果是显着的,并揭示了具有广泛异质性的水化层中的各种水行为。我们分别定义了溶解速度和角速度来量化水网络刚性和局部蛋白质灵活性。我们还观察到动态水合层扩展到超过 10 A。最后,从天然状态到熔融球状态,水合水网络松散,蛋白质局部变得更加灵活,具有更大的全局可塑性和部分展开。
更新日期:2009-08-05
中文翻译:

耦合水-蛋白质波动的蛋白质水合动力学和分子机制
蛋白质表面水化是其结构稳定性和灵活性的基础,水-蛋白质波动对生物功能至关重要。在这里,我们报告了在天然和熔融球状态下的脱脂蛋白肌红蛋白模型蛋白周围水化层中水运动的系统全局映射。通过定点诱变,我们使用内在色氨酸作为局部光学探针以单点特异性一次一个地扫描蛋白质表面。通过飞秒分辨率,我们检查了两种状态下的 16 个突变体,并观察到两种具有不同能量和时间分布的水网络弛豫。第一次水运动由局部集体氢键网络弛豫引起,发生在几皮秒内。在飞秒内在大量水中观察到的初始受阻运动,由于层中的结构化水网络集体性,受到高度抑制并显着减慢。第二次水网络松弛明确地由内部水化壳中的横向协同重排引起,发生在数十到数百皮秒内。重要的是,这种长期动力学是耦合的界面水-蛋白质运动,是这种协同波动的直接测量。这些局部蛋白质运动虽然受到高度限制,但对于帮助长时间的水网络松弛是必要的。观察到一系列水化水动力学和耦合波动与局部蛋白质的化学和结构特性的相关性。这些结果是显着的,并揭示了具有广泛异质性的水化层中的各种水行为。我们分别定义了溶解速度和角速度来量化水网络刚性和局部蛋白质灵活性。我们还观察到动态水合层扩展到超过 10 A。最后,从天然状态到熔融球状态,水合水网络松散,蛋白质局部变得更加灵活,具有更大的全局可塑性和部分展开。















































 京公网安备 11010802027423号
京公网安备 11010802027423号