Synthesis ( IF 2.2 ) Pub Date : 2021-11-22 , DOI: 10.1055/s-0041-1737276 Keith P. Reber , Priyansh D. Gujarati
|
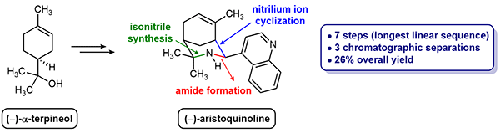
The enantioselective total synthesis of the alkaloid aristoquinoline has been achieved in seven steps and 26% overall yield. A new preparation of the useful synthetic building block (–)-α-terpinyl amine was also developed in order to avoid stoichiometric mercury reagents or azide-containing intermediates. Key steps in the optimized synthetic route include an intramolecular nitrilium ion cyclization to form the characteristic azabicyclo[3.3.1]nonane ring system and a diastereoselective reduction of the resulting imine mixture to afford the natural product. An isomer of aristoquinoline containing an exocyclic alkene was also obtained and found to exhibit unusual chromatographic and spectroscopic properties.
中文翻译:

(-)-Aristoquinoline 通过分子内腈离子环化的全合成
生物碱马里多喹啉的对映选择性全合成已通过七个步骤实现,总产率为 26%。为了避免化学计量的汞试剂或含叠氮化物的中间体,还开发了一种有用的合成结构单元 (-)-α-萜品基胺的新制备方法。优化合成路线的关键步骤包括分子内腈离子环化以形成特征氮杂双环 [3.3.1] 壬烷环系统和所得亚胺混合物的非对映选择性还原以提供天然产物。还获得了一种含有环外烯烃的马兜铃喹啉异构体,并发现其表现出不寻常的色谱和光谱特性。































 京公网安备 11010802027423号
京公网安备 11010802027423号