当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water-In-Salt LiTFSI Aqueous Electrolytes (2): Transport Properties and Li+ Dynamics Based on Molecular Dynamics Simulations
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-11-23 , DOI: 10.1021/acs.jpcb.1c07581 Yong Zhang 1, 2 , Edward J Maginn 1, 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-11-23 , DOI: 10.1021/acs.jpcb.1c07581 Yong Zhang 1, 2 , Edward J Maginn 1, 2
Affiliation
|
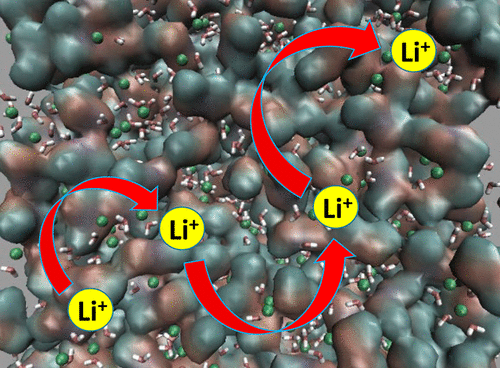
The transport properties of water-in-salt lithium bis(trifluoromethane sulfonyl)imide (LiTFSI) aqueous electrolytes were studied using classical molecular dynamics (MD) simulations. At high salt concentrations of 20 m, the calculated viscosity, self-diffusion coefficients, ionic conductivity, the inverse Haven ratio, and the Li+ apparent transference number all agree with previous experimental results quantitatively. Furthermore, analyses show that the high apparent transference number for Li+ is due to the fact that the dynamics of TFSI– decrease more quickly with increasing salt concentration than the dynamics of Li+ ions due to the formation of a TFSI– network. In addition, it was shown that the conduction of Li+ ions through the highly concentrated electrolyte occurs mainly via a hopping mechanism instead of a vehicular mechanism hypothesized in earlier studies of this system.
中文翻译:

盐包水 LiTFSI 水性电解质 (2):基于分子动力学模拟的传输特性和 Li+ 动力学
使用经典分子动力学 (MD) 模拟研究了盐包水锂双(三氟甲烷磺酰基)亚胺(LiTFSI)水性电解质的传输特性。在 20 m 的高盐浓度下,计算的粘度、自扩散系数、离子电导率、反哈文比和 Li +表观转移数都与先前的实验结果定量一致。此外,分析表明,Li +的高表观转移数是由于 TFSI –的动力学随着盐浓度的增加而下降的速度比由于 TFSI 的形成导致的 Li +离子的动力学下降得更快–网络。此外,研究表明 Li +离子通过高浓度电解质的传导主要通过跳跃机制而不是在该系统的早期研究中假设的车辆机制发生。
更新日期:2021-12-09
中文翻译:

盐包水 LiTFSI 水性电解质 (2):基于分子动力学模拟的传输特性和 Li+ 动力学
使用经典分子动力学 (MD) 模拟研究了盐包水锂双(三氟甲烷磺酰基)亚胺(LiTFSI)水性电解质的传输特性。在 20 m 的高盐浓度下,计算的粘度、自扩散系数、离子电导率、反哈文比和 Li +表观转移数都与先前的实验结果定量一致。此外,分析表明,Li +的高表观转移数是由于 TFSI –的动力学随着盐浓度的增加而下降的速度比由于 TFSI 的形成导致的 Li +离子的动力学下降得更快–网络。此外,研究表明 Li +离子通过高浓度电解质的传导主要通过跳跃机制而不是在该系统的早期研究中假设的车辆机制发生。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号