当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dimeric Copper and Lithium Thiolates: Comparison of Copper Thiolates with Their Lithium Congeners
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-11-23 , DOI: 10.1021/acs.inorgchem.1c02226 Wenxing Zou 1 , Qihao Zhu 1 , James C Fettinger 1 , Philip P Power 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-11-23 , DOI: 10.1021/acs.inorgchem.1c02226 Wenxing Zou 1 , Qihao Zhu 1 , James C Fettinger 1 , Philip P Power 1
Affiliation
|
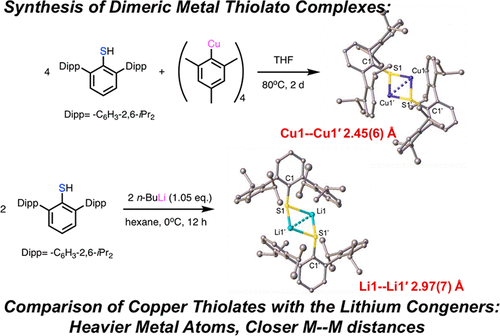
The direct reactions of the large terphenyl thiols HSAriPr4 (AriPr4= −C6H3-2,6-(C6H3-2,6-iPr2)2) and HSAriPr6 (AriPr6= −C6H3-2,6-(C6H2-2,4,6-iPr3)2) with stoichiometric amounts of mesitylcopper(I) in THF at ca. 80 °C afforded the first well-characterized dimeric copper thiolato species {CuSAriPr4}2 (1) and {CuSAriPr6}2 (2) with elimination of mesitylene. The complexes 1 and 2 were characterized by NMR and electronic spectroscopy as well as by X-ray crystallography. They have dimeric Cu2S2 core structures in which the two copper atoms are bridged by the sulfurs from the thiolato ligands and feature short Cu--Cu distances near 2.4 Å as well as a weak copper-flanking aryl ring interaction from a terphenyl substituent. The structures of the planar Cu2S2 cores bear a resemblance to the CuA site in nitrous oxide reductase in which two cysteines also bridge two copper atoms. The related dimeric Li2S2 structural motif was also observed in the lithium congeners {LiSAriPr4}2 (3) and {LiSAriPr6}2 (4) which were synthesized directly from the thiols and n-BuLi in hexanes. However, despite the very similar effective ionic radii of the Li+ (0.59 Å) and Cu+ (0.60 Å) ions, the Li--Li structures display very much longer (by more than ca. 0.5 Å) separations than the corresponding Cu--Cu distances in 1 and 2, which may be due to weaker dispersion interactions.
中文翻译:

二聚铜和硫醇锂:硫醇铜与其锂同类物的比较
大三联苯硫醇 HSAr iPr4 (Ar iPr4 = -C 6 H 3 -2,6-(C 6 H 3 -2,6-iPr 2 ) 2 ) 和 HSAr iPr6 (Ar iPr6 = -C 6 H) 的直接反应3 -2,6-(C 6 H 2 -2,4,6-iPr 3 ) 2 ) 与化学计算量的在 THF 中的甲基铜 (I) 约。80 °C 提供了第一个充分表征的二聚硫醇铜物种 {CuSAr iPr4 } 2 ( 1 ) 和 {CuSAr iPr6 }2 ( 2 ) 消除均三甲苯。配合物1和2通过核磁共振和电子光谱以及 X 射线晶体学表征。它们具有二聚体 Cu 2 S 2核心结构,其中两个铜原子由硫醇基配体的硫桥接,并具有接近 2.4 Å 的短 Cu-Cu 距离以及来自三联苯取代基的弱铜侧翼芳环相互作用. 平面Cu 2 S 2核的结构与一氧化二氮还原酶中的Cu A位点相似,其中两个半胱氨酸还桥接两个铜原子。相关的二聚体 Li 2 S 2在由硫醇和正丁基锂在己烷中直接合成的锂同源物 {LiSAr iPr4 } 2 ( 3 ) 和 {LiSAr iPr6 } 2 ( 4 )中也观察到了结构基序。然而,尽管 Li + (0.59 Å) 和 Cu + (0.60 Å) 离子的有效离子半径非常相似,但 Li-Li 结构显示出比相应的 Cu 更长(超过约 0.5 Å)的分离--Cu 距离在1和2 中,这可能是由于较弱的色散相互作用。
更新日期:2021-12-06
中文翻译:

二聚铜和硫醇锂:硫醇铜与其锂同类物的比较
大三联苯硫醇 HSAr iPr4 (Ar iPr4 = -C 6 H 3 -2,6-(C 6 H 3 -2,6-iPr 2 ) 2 ) 和 HSAr iPr6 (Ar iPr6 = -C 6 H) 的直接反应3 -2,6-(C 6 H 2 -2,4,6-iPr 3 ) 2 ) 与化学计算量的在 THF 中的甲基铜 (I) 约。80 °C 提供了第一个充分表征的二聚硫醇铜物种 {CuSAr iPr4 } 2 ( 1 ) 和 {CuSAr iPr6 }2 ( 2 ) 消除均三甲苯。配合物1和2通过核磁共振和电子光谱以及 X 射线晶体学表征。它们具有二聚体 Cu 2 S 2核心结构,其中两个铜原子由硫醇基配体的硫桥接,并具有接近 2.4 Å 的短 Cu-Cu 距离以及来自三联苯取代基的弱铜侧翼芳环相互作用. 平面Cu 2 S 2核的结构与一氧化二氮还原酶中的Cu A位点相似,其中两个半胱氨酸还桥接两个铜原子。相关的二聚体 Li 2 S 2在由硫醇和正丁基锂在己烷中直接合成的锂同源物 {LiSAr iPr4 } 2 ( 3 ) 和 {LiSAr iPr6 } 2 ( 4 )中也观察到了结构基序。然而,尽管 Li + (0.59 Å) 和 Cu + (0.60 Å) 离子的有效离子半径非常相似,但 Li-Li 结构显示出比相应的 Cu 更长(超过约 0.5 Å)的分离--Cu 距离在1和2 中,这可能是由于较弱的色散相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号