当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of Thallium(I) Oxidation by Free Chlorine in Bromide-Containing Waters: Insights into the Reactivity with Bromine Species
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-11-22 , DOI: 10.1021/acs.est.1c06901 Chengxue Ma 1, 2 , Haijun Cheng 2 , Ruixing Huang 2 , Yijie Zou 1 , Qiang He 1 , Xiaoliu Huangfu 1 , Jun Ma 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-11-22 , DOI: 10.1021/acs.est.1c06901 Chengxue Ma 1, 2 , Haijun Cheng 2 , Ruixing Huang 2 , Yijie Zou 1 , Qiang He 1 , Xiaoliu Huangfu 1 , Jun Ma 2
Affiliation
|
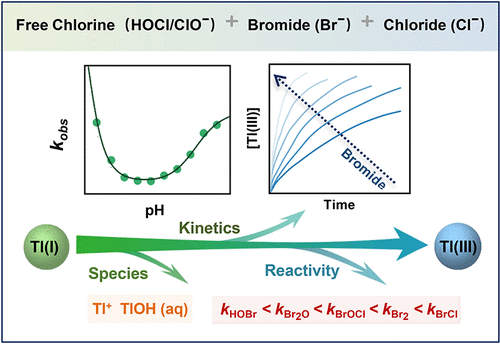
The oxidation of thallium [Tl(I)] to Tl(III) by chlorine (HOCl) is an important process changing its removal performance in water treatment. However, the role of bromide (Br–), a common constituent in natural water, in the oxidation behavior of Tl(I) during chlorination remains unknown. Our results demonstrated that Br– was cycled and acted as a catalyst to enhance the kinetics of Tl(I) oxidation by HOCl over the pH range of 5.0–9.5. Different Tl(I) species (i.e., Tl+ and TlOH(aq)) and reactive bromine species (i.e., HOBr/BrO–, BrCl, Br2O, and BrOCl) were kinetically relevant to the enhanced oxidation of Tl(I). The oxidation by free bromine species became the dominant pathway even at a low Br– level of 50 μg/L for a chlorine dose of 2 mg of Cl2/L. It was found that the reactions of Tl+/BrCl, Tl+/BrOCl, and TlOH(aq)/HOBr dominated the kinetics of Tl(I) oxidation at pH < 6.0, pH 6.0–8.0, and pH > 8.0, respectively. The species-specific rate constants for Tl+ reacting with individual bromine species were determined and decreased in the order: BrCl > Br2 > BrOCl > Br2O > HOBr. Overall, the presented results refine our knowledge regarding the species-specific reactivity of TI(I) with bromine species and will be useful for further prediction of thallium mobility in chlorinated waters containing bromide.
中文翻译:

含溴水中游离氯氧化铊 (I) 的动力学:对溴物质反应性的见解
氯 (HOCl) 将铊 [Tl(I)] 氧化为 Tl(III) 是改变其在水处理中去除性能的重要过程。然而,溴化物 (Br – ) 是天然水中的常见成分,在氯化过程中 Tl(I) 的氧化行为中的作用仍然未知。我们的结果表明,在 5.0-9.5 的 pH 范围内,Br -被循环并充当催化剂以增强 HOCl 氧化 Tl(I) 的动力学。不同的 Tl(I) 物质(即 Tl +和 TlOH(aq))和活性溴物质(即 HOBr/BrO -、BrCl、Br 2 O 和 BrOCl)与 Tl(I) 的增强氧化具有动力学相关性. 即使在低 Br 条件下,游离溴物质的氧化也成为主要途径-对于 2 mg Cl 2 /L的氯剂量,浓度为 50 μg/ L。发现 Tl + /BrCl、Tl + /BrOCl 和 TlOH(aq)/HOBr 的反应分别在 pH < 6.0、pH 6.0-8.0 和 pH > 8.0 时主导 Tl(I) 氧化的动力学。Tl +与单个溴物种反应的物种特异性速率常数按以下顺序确定并降低:BrCl > Br 2 > BrOCl > Br 2 O > HOBr。总体而言,所呈现的结果完善了我们关于 TI(I) 与溴物种的物种特异性反应性的知识,并将有助于进一步预测铊在含溴化物的氯化水中的迁移率。
更新日期:2022-01-18
中文翻译:

含溴水中游离氯氧化铊 (I) 的动力学:对溴物质反应性的见解
氯 (HOCl) 将铊 [Tl(I)] 氧化为 Tl(III) 是改变其在水处理中去除性能的重要过程。然而,溴化物 (Br – ) 是天然水中的常见成分,在氯化过程中 Tl(I) 的氧化行为中的作用仍然未知。我们的结果表明,在 5.0-9.5 的 pH 范围内,Br -被循环并充当催化剂以增强 HOCl 氧化 Tl(I) 的动力学。不同的 Tl(I) 物质(即 Tl +和 TlOH(aq))和活性溴物质(即 HOBr/BrO -、BrCl、Br 2 O 和 BrOCl)与 Tl(I) 的增强氧化具有动力学相关性. 即使在低 Br 条件下,游离溴物质的氧化也成为主要途径-对于 2 mg Cl 2 /L的氯剂量,浓度为 50 μg/ L。发现 Tl + /BrCl、Tl + /BrOCl 和 TlOH(aq)/HOBr 的反应分别在 pH < 6.0、pH 6.0-8.0 和 pH > 8.0 时主导 Tl(I) 氧化的动力学。Tl +与单个溴物种反应的物种特异性速率常数按以下顺序确定并降低:BrCl > Br 2 > BrOCl > Br 2 O > HOBr。总体而言,所呈现的结果完善了我们关于 TI(I) 与溴物种的物种特异性反应性的知识,并将有助于进一步预测铊在含溴化物的氯化水中的迁移率。































 京公网安备 11010802027423号
京公网安备 11010802027423号