Mini-Reviews in Organic Chemistry ( IF 1.9 ) Pub Date : 2021-11-30 , DOI: 10.2174/1570193x18666210203160106
Mehwish Iftikhar 1 , Min Zhou 1 , Yinghong Lu 1
|
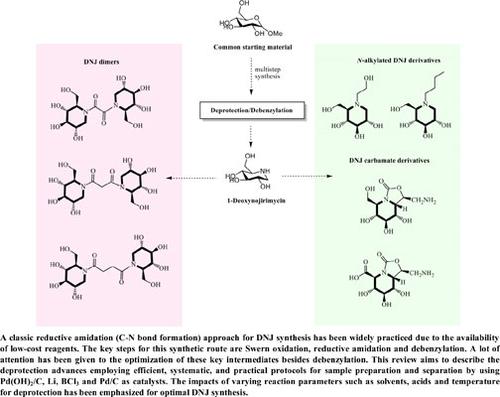
A classic reductive amidation/amination (C-N bond formation) approach for DNJ synthesis has been widely practiced due to the availability of low-cost reagents. The key steps for this synthetic route are Swern oxidation, reductive amidation/amination, and debenzylation. A lot of attention has been given to the optimization of these key intermediates apart from debenzylation. This review aims to describe the deprotection advances employing efficient, systematic, and practical protocols for sample preparation and separation by using Pd(OH)2/C, Li, BCl3 and Pd/C as catalysts. The impact of varying reaction parameters such as solvents, acids and temperature for deprotection was emphasized for optimal DNJ synthesis.
中文翻译:

获得 1-脱氧野尻霉素 (DNJ) 的脱苄基方法概述
由于低成本试剂的可用性,用于 DNJ 合成的经典还原酰胺化/胺化(CN 键形成)方法已被广泛采用。该合成路线的关键步骤是 Swern 氧化、还原酰胺化/胺化和脱苄基。除了脱苄基外,对这些关键中间体的优化也给予了很多关注。本综述旨在描述使用 Pd(OH) 2 /C、Li、BCl 3和 Pd/C 作为催化剂,采用高效、系统和实用的协议进行样品制备和分离的脱保护进展。为了优化 DNJ 合成,强调了不同反应参数(例如溶剂、酸和脱保护温度)的影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号