当前位置:
X-MOL 学术
›
J. Diabetes Investig.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preserved pharmacokinetics and pharmacodynamics of insulin degludec and liraglutide when administered as insulin degludec/liraglutide in a Chinese population
Journal of Diabetes Investigation ( IF 3.1 ) Pub Date : 2021-11-19 , DOI: 10.1111/jdi.13716 Hongzhong Liu 1, 2 , Bin Luo 3 , Xia Chen 1, 4 , Steen H Ingwersen 5 , Ting Jia 5 , Lisbeth Vestergård Jacobsen 5 , Pei Hu 1, 2
Journal of Diabetes Investigation ( IF 3.1 ) Pub Date : 2021-11-19 , DOI: 10.1111/jdi.13716 Hongzhong Liu 1, 2 , Bin Luo 3 , Xia Chen 1, 4 , Steen H Ingwersen 5 , Ting Jia 5 , Lisbeth Vestergård Jacobsen 5 , Pei Hu 1, 2
Affiliation
|
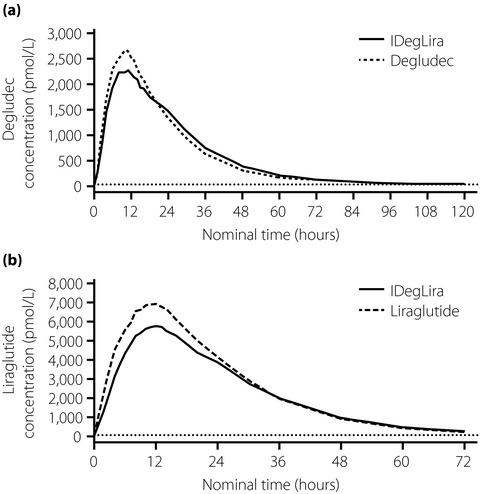
We report the findings of a single-dose, randomized, three-period cross-over, clinical trial in healthy Chinese individuals (n = 24) comparing the pharmacokinetics of insulin degludec/liraglutide (IDegLira) with its individual components. Furthermore, we report a population pharmacokinetic analysis of a 26-week, phase III, treat-to-target, randomized trial of 720 Chinese individuals with type 2 diabetes. Participants were randomized to IDegLira, degludec or liraglutide, all once daily with metformin. The pharmacokinetic profiles of IDegLira were similar to its individual components. Dose proportionality was indicated for both IDegLira components. Although there were no relevant covariate effects on degludec exposure, liraglutide exposure was inversely correlated with bodyweight. In conclusion, for the Chinese population, the pharmacokinetics of the fixed-ratio combination IDegLira is similar to that of its individual components.
更新日期:2021-11-19
















































 京公网安备 11010802027423号
京公网安备 11010802027423号