当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent-Free Mechanochemical Diaza-Cope Rearrangement
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-11-19 , DOI: 10.1021/acssuschemeng.1c04459
Wenxian Ma 1, 2, 3 , Yan Liu 1 , Na Yu 1 , KaKing Yan 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-11-19 , DOI: 10.1021/acssuschemeng.1c04459
Wenxian Ma 1, 2, 3 , Yan Liu 1 , Na Yu 1 , KaKing Yan 1
Affiliation
|
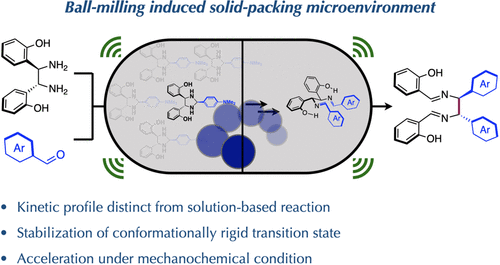
Chiral vicinal diamines represent an important class of organic building blocks that play essential roles in various fields in chemical and material sciences. Their syntheses have continued to attract attention to enhance their yield, selectivity, and scope, but all of the procedures reported so far in the literature are solvent-based reactions. We herein report the first example of mechanochemical diaza-Cope rearrangement to convert a wide variety of aldehyde substrates into chiral diimines, which upon hydrolysis lead to the formation of chiral vicinal diamines. This solvent-free protocol is highlighted with high yield, fast reaction, and simple synthetic operation with commercially available precursors and is easily scalable to gram quantity. Mechanistic investigations revealed a distinct reaction profile for mechanochemical-induced reactions compared to solution-based reactions. Reaction time course studies showed that the diaza-Cope rearrangement step, which requires a highly ordered six-membered chair-like TS, is rapid in mechanochemical-induced reactions. This enhanced rate stems from the formation of effective solid-state packing in mechanochemical reaction settings. The powder X-ray diffraction analysis of the milled sample confirmed the formation of a new crystalline phase during the mechanochemical reaction, which leads to continuation of the reaction even after ball milling is stopped. Furthermore, we developed a one-pot two-step procedure to synthesize chiral salen metal complexes, a “privileged” chiral catalyst, directly from arylaldehydes without the need for isolating the rearrangement products. From a practicality perspective, this work demonstrates a mechanochemical synthesis of chiral vicinal diamines that can be readily adapted in academic laboratories and, potentially, in industrial settings.
中文翻译:

无溶剂机械化学重氮基重排
手性连二胺代表了一类重要的有机结构单元,在化学和材料科学的各个领域都发挥着重要作用。它们的合成继续引起人们的注意,以提高它们的产率、选择性和范围,但迄今为止文献中报道的所有程序都是基于溶剂的反应。我们在此报告了机械化学重氮-Cope 重排的第一个例子,将各种醛底物转化为手性二亚胺,水解后形成手性邻位二胺。这种无溶剂方案的突出特点是产量高、反应速度快、合成操作简单,可使用市售前体,并可轻松扩展到克数量。机理研究表明,与基于溶液的反应相比,机械化学诱导的反应具有不同的反应特征。反应时程研究表明,二氮杂-科普重排步骤需要高度有序的六元椅子状 TS,在机械化学诱导的反应中是快速的。这种提高的速率源于在机械化学反应环境中形成有效的固态堆积。研磨样品的粉末 X 射线衍射分析证实了在机械化学反应过程中形成了新的结晶相,这导致即使在球磨停止后反应继续进行。此外,我们开发了一种一锅两步法来合成手性salen金属配合物,一种“特权”手性催化剂,直接从芳醛中分离,无需分离重排产物。从实用性的角度来看,这项工作展示了手性连二胺的机械化学合成,可以很容易地在学术实验室中进行调整,并且可能在工业环境中进行调整。
更新日期:2021-12-06
中文翻译:

无溶剂机械化学重氮基重排
手性连二胺代表了一类重要的有机结构单元,在化学和材料科学的各个领域都发挥着重要作用。它们的合成继续引起人们的注意,以提高它们的产率、选择性和范围,但迄今为止文献中报道的所有程序都是基于溶剂的反应。我们在此报告了机械化学重氮-Cope 重排的第一个例子,将各种醛底物转化为手性二亚胺,水解后形成手性邻位二胺。这种无溶剂方案的突出特点是产量高、反应速度快、合成操作简单,可使用市售前体,并可轻松扩展到克数量。机理研究表明,与基于溶液的反应相比,机械化学诱导的反应具有不同的反应特征。反应时程研究表明,二氮杂-科普重排步骤需要高度有序的六元椅子状 TS,在机械化学诱导的反应中是快速的。这种提高的速率源于在机械化学反应环境中形成有效的固态堆积。研磨样品的粉末 X 射线衍射分析证实了在机械化学反应过程中形成了新的结晶相,这导致即使在球磨停止后反应继续进行。此外,我们开发了一种一锅两步法来合成手性salen金属配合物,一种“特权”手性催化剂,直接从芳醛中分离,无需分离重排产物。从实用性的角度来看,这项工作展示了手性连二胺的机械化学合成,可以很容易地在学术实验室中进行调整,并且可能在工业环境中进行调整。




































 京公网安备 11010802027423号
京公网安备 11010802027423号