当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DFT Studies of the Selective C–O Hydrogenolysis and Ring-Opening of Biomass-Derived Tetrahydrofurfuryl Alcohol over Rh(111) surfaces
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-08-18 00:00:00 , DOI: 10.1021/acs.jpcc.6b05026 Jing Guan 1 , Jing Li 2 , Yifeng Yu 2 , Xindong Mu 1 , Aibing Chen 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-08-18 00:00:00 , DOI: 10.1021/acs.jpcc.6b05026 Jing Guan 1 , Jing Li 2 , Yifeng Yu 2 , Xindong Mu 1 , Aibing Chen 2
Affiliation
|
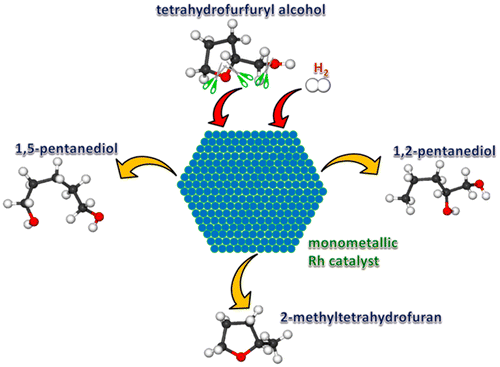
Tetrahydrofurfuryl alcohol (THFA) has been identified as a platform chemical of interest because of its production from biomass. It can be converted into valuable alcohols and ethers by selective hydrogenation/hydrogenolysis reaction over Rh-based metal catalysts. To better understand the chemistry of THFA, the reaction energies and the corresponding energy barriers of selective C–O bond hydrogenolysis and ring-opening of THFA on Rh(111) for the formation of 2-methyltetrahydrofuran (2-MeTHF), 1,5-pentanediol (1,5-PeD), and 1,2-pentanediol (1,2-PeD) were studied using density functional theory (DFT) calculations. The results indicate that starting from THFA to produce 2-MeTHF, the direct C–O bond cleavage of the CH2OH group is not favored. Alternatively and more preferentially, the reaction occurs through the initial activation of C–H bond on the side chain, followed by dehydroxylation and hydrogenation. On the other hand, in the metal catalyzed ring-opening process of THFA to 1,5-PeD and 1,2-PeD, the first dehydrogenation of secondary CH–O or primary CH2–O moiety in the ring decreases the barriers of the subsequent C–O bond dissociation. Moreover, the energy span theory shows that the ring-opening at the sterically less-hindered primary C–O bond exhibits a lower effective barrier than that for ring-opening at the more sterically hindered secondary C–O bond, as well as hydrogenolysis at the side CH2OH chain, suggesting that the formation of 1,2-PeD is much kinetically favored than the formation of 1,5-PeD and 2-MeTHF. Our theoretical results give a good explanation for the experimental fact that 1,2-PeD was the dominant product observed on unprompted Rh/SiO2.
中文翻译:

DFT研究在Rh(111)表面上选择性C–O氢解和生物质衍生的四氢糠醇的开环
由于四氢糠醇(THFA)是由生物质生产的,因此已被视为重要的平台化学品。通过在Rh基金属催化剂上进行选择性加氢/氢解反应,可以将其转化为有价值的醇和醚。为了更好地了解THFA的化学性质,在Rh(111)上形成2-甲基四氢呋喃(2-MeTHF),1,5的选择性C–O键氢解和THFA的开环反应的能量以及相应的能垒使用密度泛函理论(DFT)计算研究了-戊二醇(1,5-PeD)和1,2-戊二醇(1,2-PeD)。结果表明,从THFA开始生成2-MeTHF,即CH 2的直接C–O键裂解OH基团是不受欢迎的。另外,更优先的是,反应通过侧链上C–H键的初始活化发生,然后进行脱羟基和氢化反应。另一方面,在THFA的金属催化开环过程中,生成1,5-PeD和1,2-PeD的过程是,环中仲CH–O或伯CH 2 –O部分的第一次脱氢降低了CHFA的势垒。随后的C–O键解离。此外,能量跨度理论表明,在空间上受阻较小的一级C-O键处的开环所表现出的有效势垒低于在空间上受阻的二级C-O键处的开环,以及在氢键处的氢解作用。侧面CH 2OH链,这表明1,2-PeD的形成比1,5-PeD和2-MeTHF的形成在动力学上更受青睐。我们的理论结果很好地解释了实验事实,即1,2-PeD是在无提示Rh / SiO 2上观察到的主要产物。
更新日期:2016-08-18
中文翻译:

DFT研究在Rh(111)表面上选择性C–O氢解和生物质衍生的四氢糠醇的开环
由于四氢糠醇(THFA)是由生物质生产的,因此已被视为重要的平台化学品。通过在Rh基金属催化剂上进行选择性加氢/氢解反应,可以将其转化为有价值的醇和醚。为了更好地了解THFA的化学性质,在Rh(111)上形成2-甲基四氢呋喃(2-MeTHF),1,5的选择性C–O键氢解和THFA的开环反应的能量以及相应的能垒使用密度泛函理论(DFT)计算研究了-戊二醇(1,5-PeD)和1,2-戊二醇(1,2-PeD)。结果表明,从THFA开始生成2-MeTHF,即CH 2的直接C–O键裂解OH基团是不受欢迎的。另外,更优先的是,反应通过侧链上C–H键的初始活化发生,然后进行脱羟基和氢化反应。另一方面,在THFA的金属催化开环过程中,生成1,5-PeD和1,2-PeD的过程是,环中仲CH–O或伯CH 2 –O部分的第一次脱氢降低了CHFA的势垒。随后的C–O键解离。此外,能量跨度理论表明,在空间上受阻较小的一级C-O键处的开环所表现出的有效势垒低于在空间上受阻的二级C-O键处的开环,以及在氢键处的氢解作用。侧面CH 2OH链,这表明1,2-PeD的形成比1,5-PeD和2-MeTHF的形成在动力学上更受青睐。我们的理论结果很好地解释了实验事实,即1,2-PeD是在无提示Rh / SiO 2上观察到的主要产物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号