Tetrahedron ( IF 2.1 ) Pub Date : 2021-11-18 , DOI: 10.1016/j.tet.2021.132563 Kseniia Yu Titenkova 1, 2 , Alexander V. Shaferov 1 , Alexander A. Larin 1 , Margarita A. Epishina 1 , Alexander S. Kulikov 1 , Ivan V. Ananyev 3, 4 , Leonid L. Fershtat 1
|
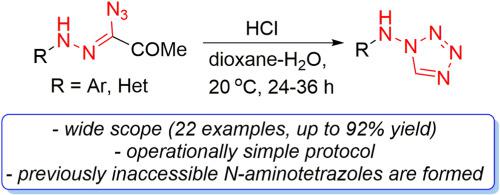
A general method for the synthesis of a series of previously unknown N-(heteroaryl)- and N-(aryl)aminotetrazoles through the tandem electrocyclization/hydrolysis of azidohydrazones was accomplished. The described protocol was suitable for a wide array of the target N-substituted aminotetrazoles which were prepared in good to high yields under smooth reaction conditions. Importantly, the presented approach is considered as a direct route to 1,2,5-oxadiazole-substituted aminotetrazole derivatives in good and high yields and may be realized on a gram scale. Therefore, facile synthesis of N-(heteroaryl)- and N-(aryl)aminotetrazoles unveils novel opportunities to study application prospectives of these compounds as functional materials or drug candidates.
中文翻译:

串联酸促进分子内叠氮化物-腙电环化/水解方法合成N-氨基四唑
完成了通过叠氮腙串联电环化/水解合成一系列以前未知的N- (杂芳基)-和N- (芳基)氨基四唑的通用方法。所描述的协议适用于广泛的目标N-取代氨基四唑,这些目标在平稳反应条件下以良好到高产率制备。重要的是,所提出的方法被认为是以良好和高产率获得 1,2,5-恶二唑取代的氨基四唑衍生物的直接途径,并且可以在克规模上实现。因此,容易合成N- (杂芳基)-和N-(芳基)氨基四唑为研究这些化合物作为功能材料或候选药物的应用前景提供了新的机会。































 京公网安备 11010802027423号
京公网安备 11010802027423号