当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fast Generation of Hydroxyl Radicals by Rerouting the Electron Transfer Pathway via Constructed Chemical Channels during the Photo-Electro-Reduction of Oxygen
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-11-18 , DOI: 10.1021/acs.est.1c06368 Jinxing Zhang 1 , Zhaoyu Zhou 1 , Zhiyuan Feng 1 , Hongying Zhao 1 , Guohua Zhao 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-11-18 , DOI: 10.1021/acs.est.1c06368 Jinxing Zhang 1 , Zhaoyu Zhou 1 , Zhiyuan Feng 1 , Hongying Zhao 1 , Guohua Zhao 1
Affiliation
|
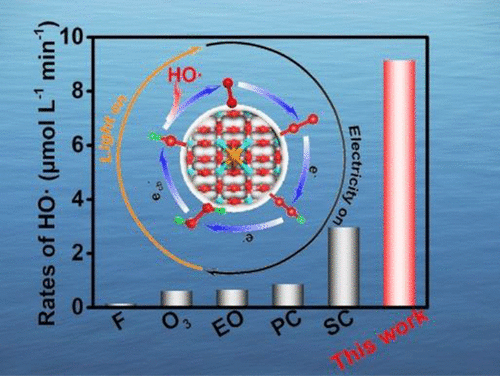
A strategy for the fast generation of hydroxyl radicals (HO·) via photo-electro-reduction of oxygen by rerouting the electron transfer pathway was proposed. The rate-determining step of HO· production is the formation of H2O2 and the simultaneous reduction of H2O2. Engineering of F-TiO2 with single atom Pd bonded with four F and two O atoms favored the electrocatalytic 2-electron oxygen reduction to H2O2 with as high as 99% selectivity, while the additional channel bond HO–O···Pd–F–TiO2 facilitates the photogenerated electron transfer from the conduction band to single atom Pd to reduce Pd···O–OH to HO·. The optimized HO· production rate is 9.18 μ mol L–1 min–1, which is 2.6–52.5 times higher than that in traditional advanced oxidation processes. In the application of wastewater treatment, this proposed photoelectrocatalytic oxygen reduction method, respectively, shows fast kinetics of 0.324 and 0.175 min–1 for removing bisphenol A and acetaminophen. Around 93.2% total organic carbon and 99.3% acute toxicity removal were achieved. Additionally, the degradation efficiency was less affected by the water source and pH value because of the evitable usage of metallic active sites. This work represents a fundamental investigation on the generation rate of HO·, which would pave the way for the future development of photoelectrocatalytic technologies for water purification.
中文翻译:

在氧的光电还原过程中,通过构建的化学通道改变电子转移路径,快速生成羟基自由基
提出了一种通过改变电子转移路径通过光电还原氧来快速产生羟基自由基(HO·)的策略。HO·产生的速率决定步骤是H 2 O 2 的形成和H 2 O 2的同时还原。单原子Pd键合四个F和两个O原子的F-TiO 2工程有利于电催化2电子氧还原为H 2 O 2,选择性高达99%,而额外的通道键HO-O··· Pd-F-TiO 2促进光生电子从导带转移到单原子 Pd 以将 Pd···O-OH 还原为 HO·。优化后的HO·产率为9.18 μ mol L –1 min –1,是传统高级氧化工艺的2.6-52.5倍。在废水处理应用中,本文提出的光电催化氧还原方法分别表现出 0.324 和 0.175 min –1的快速动力学用于去除双酚A和对乙酰氨基酚。实现了约 93.2% 的总有机碳和 99.3% 的急性毒性去除。此外,由于不可避免地使用金属活性位点,降解效率受水源和 pH 值的影响较小。这项工作代表了对HO·生成速率的基本研究,这将为未来水净化光电催化技术的发展铺平道路。
更新日期:2022-01-18
中文翻译:

在氧的光电还原过程中,通过构建的化学通道改变电子转移路径,快速生成羟基自由基
提出了一种通过改变电子转移路径通过光电还原氧来快速产生羟基自由基(HO·)的策略。HO·产生的速率决定步骤是H 2 O 2 的形成和H 2 O 2的同时还原。单原子Pd键合四个F和两个O原子的F-TiO 2工程有利于电催化2电子氧还原为H 2 O 2,选择性高达99%,而额外的通道键HO-O··· Pd-F-TiO 2促进光生电子从导带转移到单原子 Pd 以将 Pd···O-OH 还原为 HO·。优化后的HO·产率为9.18 μ mol L –1 min –1,是传统高级氧化工艺的2.6-52.5倍。在废水处理应用中,本文提出的光电催化氧还原方法分别表现出 0.324 和 0.175 min –1的快速动力学用于去除双酚A和对乙酰氨基酚。实现了约 93.2% 的总有机碳和 99.3% 的急性毒性去除。此外,由于不可避免地使用金属活性位点,降解效率受水源和 pH 值的影响较小。这项工作代表了对HO·生成速率的基本研究,这将为未来水净化光电催化技术的发展铺平道路。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号