当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Prowess of Photogenerated Amine Radical Cations in Cascade Reactions: From Carbocycles to Heterocycles
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2016-08-18 00:00:00 , DOI: 10.1021/acs.accounts.6b00263
Scott A. Morris 1 , Jiang Wang 1 , Nan Zheng 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2016-08-18 00:00:00 , DOI: 10.1021/acs.accounts.6b00263
Scott A. Morris 1 , Jiang Wang 1 , Nan Zheng 1
Affiliation
|
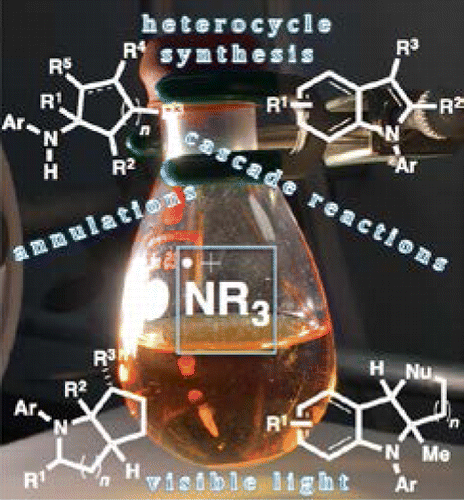
Cascade reactions represent a class of ideal organic reactions because they empower efficiency, elegance, and novelty. However, development of cascade reactions remains a daunting task for synthetic chemists. Radicals are known to be well suited for cascade reactions. Compared with widely used carbon-based radicals, nitrogen-based radicals, such as neutral aminyl radicals and protonated aminyl radicals (amine radical cations), are underutilized, although they are behind some notable synthetic methods such as the Hofmann–Löffler–Freytag reaction. The constraint on their usage is generally attributed to the limited number of available stable precursors. Since amine radical cations offer increased reactivity and selectivity in chemical transformations compared with neutral aminyl radicals, their generation is of utmost importance. Recently, a surge of reports has been revealed using visible light photoredox catalysis. It has been demonstrated that amines can act as an electron donor in a reductive quenching cycle while the amine itself is oxidized to the amine radical cation. Although a number of methods exist to generate amine radical cations, the photochemical formation of these species offers many practical advantages.
中文翻译:

级联反应中光生胺自由基阳离子的能力:从碳环化合物到杂环化合物
级联反应代表一类理想的有机反应,因为它们可以提高效率,提高优雅度和新颖性。然而,级联反应的发展对于合成化学家来说仍然是艰巨的任务。自由基是非常适合级联反应的。与广泛使用的碳基自由基相比,氮基自由基,例如中性氨基自由基和质子化氨基自由基(胺自由基阳离子),尽管它们落后于一些著名的合成方法,例如霍夫曼-勒夫勒-弗雷塔格反应,但并未得到充分利用。对它们的使用的限制通常归因于可用稳定前体的数量有限。与中性氨基自由基相比,由于胺自由基阳离子在化学转化中具有更高的反应性和选择性,因此其产生至关重要。最近,使用可见光光氧化还原催化已发现大量报告。已经证明胺可以在还原淬灭循环中充当电子给体,同时胺本身被氧化成胺自由基阳离子。尽管存在许多产生胺自由基阳离子的方法,但是这些物质的光化学形成提供了许多实际的优点。
更新日期:2016-08-18
中文翻译:

级联反应中光生胺自由基阳离子的能力:从碳环化合物到杂环化合物
级联反应代表一类理想的有机反应,因为它们可以提高效率,提高优雅度和新颖性。然而,级联反应的发展对于合成化学家来说仍然是艰巨的任务。自由基是非常适合级联反应的。与广泛使用的碳基自由基相比,氮基自由基,例如中性氨基自由基和质子化氨基自由基(胺自由基阳离子),尽管它们落后于一些著名的合成方法,例如霍夫曼-勒夫勒-弗雷塔格反应,但并未得到充分利用。对它们的使用的限制通常归因于可用稳定前体的数量有限。与中性氨基自由基相比,由于胺自由基阳离子在化学转化中具有更高的反应性和选择性,因此其产生至关重要。最近,使用可见光光氧化还原催化已发现大量报告。已经证明胺可以在还原淬灭循环中充当电子给体,同时胺本身被氧化成胺自由基阳离子。尽管存在许多产生胺自由基阳离子的方法,但是这些物质的光化学形成提供了许多实际的优点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号