当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chitin Deacetylase as a Biocatalyst for the Selective N-Acylation of Chitosan Oligo- and Polymers
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-11-16 , DOI: 10.1021/acscatal.1c04472 Max Linhorst 1 , Jasper Wattjes 1 , Bruno M. Moerschbacher 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-11-16 , DOI: 10.1021/acscatal.1c04472 Max Linhorst 1 , Jasper Wattjes 1 , Bruno M. Moerschbacher 1
Affiliation
|
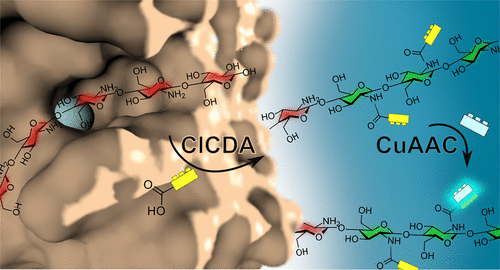
Chitosans, derived from the abundant natural resource chitin, are among the most versatile and promising functional biopolymers due to their unique physicochemical properties and biological activities. These can be further improved or modified by various functionalizations, targeting both the hydroxy and amino groups. However, the chemical routes used for functionalization typically use harsh chemicals, increasing the ecological footprint of the products, tend to yield low degrees of substitution, and often lack chemoselectivity. We here report on an alternative, biocatalytic route of chitosan N-acylation using a recombinant chitin deacetylase (CDA). These enzymes are known for their ability to chemo- and regioselectively N-acetylate glucosamine oligomers, and they were recently shown to also exhibit this reverse activity toward polyglucosamine, yielding partially acetylated chitosan polymers with a nonrandom pattern of acetylation. As chitin deacetylases can possess a certain cosubstrate promiscuity, we explored the ability of a CDA from the fungus Colletotrichum lindemuthianum (ClCDA) to N-acylate glucosamine tetramers and polyglucosamines using a range of small carboxylic acids as cosubstrates. The resulting tetramers were analyzed using ultra-high-performance hydrophilic liquid chromatography-tandem mass spectrometry (UHPLC-HILIC-MS), and the kinetic parameters of the acylation reactions thus determined gave deeper insight into the limitation of the cosubstrate scope of ClCDA. Using polyglucosamines as substrates, we obtained N-propiolated chitosan polymers with high fractions of substitution of 0.7. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) then yielded a fluorescence-labeled polymer, providing proof-of-principle for click functionalization of chitosans using this chemoenzymatic approach. Given the known regioselectivity of chitin deacetylases, which is retained during reverse N-acetylation, this process might give access to a broad variety of functionalized chitosans with nonrandom substitution patterns.
中文翻译:

几丁质脱乙酰酶作为壳聚糖寡聚物和聚合物选择性 N-酰化的生物催化剂
壳聚糖来源于丰富的自然资源甲壳素,由于其独特的理化性质和生物活性,是用途最广泛、最有前途的功能性生物聚合物之一。这些可以通过针对羟基和氨基的各种功能化进一步改进或修饰。然而,用于功能化的化学路线通常使用刺激性化学品,增加了产品的生态足迹,往往产生低取代度,并且通常缺乏化学选择性。我们在这里报告了使用重组几丁质脱乙酰酶 (CDA)进行壳聚糖N-酰化的替代生物催化途径。这些酶以其化学和区域选择性N-乙酰化葡糖胺低聚物,并且最近显示它们也表现出对聚葡糖胺的这种反向活性,产生具有非随机乙酰化模式的部分乙酰化壳聚糖聚合物。由于几丁质脱乙酰酶可以具有一定的共底物混杂性,我们探索了从真菌炭疽病菌(ClCDA) 到N的 CDA 的能力-使用一系列小羧酸作为共底物酰化葡糖胺四聚体和聚葡糖胺。使用超高效亲水液相色谱-串联质谱 (UHPLC-HILIC-MS) 分析所得四聚体,由此确定的酰化反应动力学参数更深入地了解 ClCDA 共底物范围的局限性。使用聚葡糖胺作为底物,我们获得了具有 0.7 的高取代分数的N-丙炔化壳聚糖聚合物。然后,铜催化的叠氮化物-炔环加成 (CuAAC) 产生了一种荧光标记的聚合物,为使用这种化学酶促方法对壳聚糖进行点击功能化提供了原理证明。鉴于几丁质脱乙酰酶的已知区域选择性,在逆转过程中保留N-乙酰化,这个过程可能会提供多种具有非随机取代模式的功能化壳聚糖。
更新日期:2021-12-03
中文翻译:

几丁质脱乙酰酶作为壳聚糖寡聚物和聚合物选择性 N-酰化的生物催化剂
壳聚糖来源于丰富的自然资源甲壳素,由于其独特的理化性质和生物活性,是用途最广泛、最有前途的功能性生物聚合物之一。这些可以通过针对羟基和氨基的各种功能化进一步改进或修饰。然而,用于功能化的化学路线通常使用刺激性化学品,增加了产品的生态足迹,往往产生低取代度,并且通常缺乏化学选择性。我们在这里报告了使用重组几丁质脱乙酰酶 (CDA)进行壳聚糖N-酰化的替代生物催化途径。这些酶以其化学和区域选择性N-乙酰化葡糖胺低聚物,并且最近显示它们也表现出对聚葡糖胺的这种反向活性,产生具有非随机乙酰化模式的部分乙酰化壳聚糖聚合物。由于几丁质脱乙酰酶可以具有一定的共底物混杂性,我们探索了从真菌炭疽病菌(ClCDA) 到N的 CDA 的能力-使用一系列小羧酸作为共底物酰化葡糖胺四聚体和聚葡糖胺。使用超高效亲水液相色谱-串联质谱 (UHPLC-HILIC-MS) 分析所得四聚体,由此确定的酰化反应动力学参数更深入地了解 ClCDA 共底物范围的局限性。使用聚葡糖胺作为底物,我们获得了具有 0.7 的高取代分数的N-丙炔化壳聚糖聚合物。然后,铜催化的叠氮化物-炔环加成 (CuAAC) 产生了一种荧光标记的聚合物,为使用这种化学酶促方法对壳聚糖进行点击功能化提供了原理证明。鉴于几丁质脱乙酰酶的已知区域选择性,在逆转过程中保留N-乙酰化,这个过程可能会提供多种具有非随机取代模式的功能化壳聚糖。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号