European Polymer Journal ( IF 5.8 ) Pub Date : 2021-11-17 , DOI: 10.1016/j.eurpolymj.2021.110880
Fatima Hammoud 1, 2, 3 , Akram Hijazi 3 , Sylvain Duval 4 , Jacques Lalevée 1, 2 , Frédéric Dumur 5
|
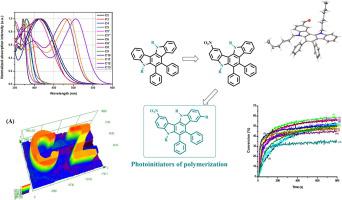
5,12-Dihydroindolo[3,2-a]carbazole is a polycyclic structure combining in its scaffold two carbazole moieties sharing a fused aromatic ring. Since the pioneering works reported in 2019 on this structure concerning the substitution of the two carbazoles by similar functional groups, no recent investigation has been devoted to asymmetrize the substitution of the two carbazoles. In this work, a series of 13 compounds based on the 5,12-dihydroindolo[3,2-a]carbazole scaffold is presented. By carefully controlling the reaction conditions, functional groups such as a formyl, a nitro or an acetyl group could be selectively introduced on one of the two carbazoles. By combining X-ray diffraction and 2D NMR experiments, the higher reactivity of one of the two carbazoles could be clearly evidenced, enabling to generate asymmetrically substituted structures. Thanks to this unprecedented approach on the 5,12-dihydroindolo[3,2-a]carbazole scaffold, the absorption of the resulting dyes could be shifted towards the visible range whereas the parent structure (5,12-dihexyl-6,7-diphenyl-5,12-dihydroindolo[3,2-a]carbazole) exhibits a strongly UV-centered absorption. In light of their visible light absorption properties, several dyes have been examined as photoinitiators of polymerization activable at 405 nm and under low light intensity in two-component photoinitiating systems for the free radical polymerization of acrylates or the cationic polymerization of epoxides. Chemical mechanisms supporting the different polymerization processes have been fully elucidated by combining several techniques including cyclic voltammetry, UV-visible absorption and photoluminescence spectroscopy as well as photolysis experiments.
中文翻译:

5,12-二氢吲哚并[3,2-a]咔唑:一种用于设计聚合可见光光引发剂的有前途的支架
5,12-二氢吲哚并[3,2- a ]咔唑是一种多环结构,在其支架中结合了共享稠合芳环的两个咔唑部分。自从 2019 年关于这种结构的开创性工作报道了两个咔唑被相似的官能团取代以来,最近没有专门研究两个咔唑的不对称取代。在这项工作中,基于 5,12-二氢吲哚[3,2- a]咔唑支架。通过仔细控制反应条件,可以在两种咔唑之一上选择性地引入甲酰基、硝基或乙酰基等官能团。通过结合 X 射线衍射和 2D NMR 实验,可以清楚地证明两种咔唑之一的更高反应性,从而能够生成不对称取代的结构。由于这种对 5,12-二氢吲哚并[3,2- a ]咔唑支架的前所未有的方法,所得染料的吸收可以向可见光范围转移,而母体结构(5,12-二己基-6,7-二苯基-5,12-二氢吲哚[3,2- a]咔唑)表现出强烈的以紫外线为中心的吸收。鉴于它们的可见光吸收特性,已经研究了几种染料作为聚合的光引发剂,可在 405 nm 和低光强度下在双组分光引发体系中用于丙烯酸酯的自由基聚合或环氧化物的阳离子聚合。通过结合包括循环伏安法、紫外-可见吸收和光致发光光谱以及光解实验在内的多种技术,已经充分阐明了支持不同聚合过程的化学机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号