Medicinal Chemistry ( IF 1.9 ) Pub Date : 2021-11-30 , DOI: 10.2174/1573406416666200817160708 Haicheng Liu 1 , Yushi Futamura 2 , Honghai Wu 1 , Aki Ishiyama 3 , Taotao Zhang 1 , Tao Shi 1 , Qunxiong Zheng 4 , Masato Iwatsuki 3 , Satoshi Ōmura 3 , Hongbin Zou 1 , Hiroyuki Osada 2
|
Background: Malaria is one of the most devastating parasitic diseases, yet the discovery of antimalarial agents remains profoundly challenging. Very few new antimalarials have been developed in the past 50 years, while the emergence of drug-resistance continues to appear.
Objective: This study focuses on the discovery, design, synthesis, and antimalarial evaluation of 3- cinnamamido-N-substituted benzamides.
Methods: In this study, a screening of our compound library was carried out against the multidrugsensitive Plasmodium falciparum 3D7 strain. Derivatives of the hit were designed, synthesized and tested against P. falciparum 3D7 and the in vivo antimalarial activity of the most active compounds was evaluated using the method of Peters’ 4-day suppressive test.
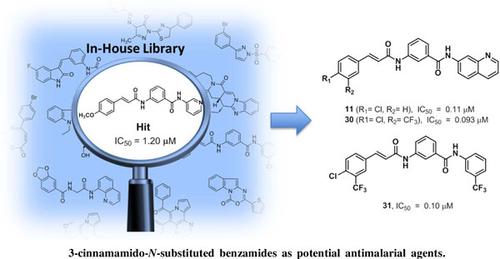
Results: The retrieved hit compound 1 containing a 3-cinnamamido-N-substituted benzamide skeleton showed moderate antimalarial activity (IC50 = 1.20 μM) for the first time. A series of derivatives were then synthesized through a simple four-step workflow, and half of them exhibited slightly better antimalarial effect than the precursor 1 during the subsequent in vitro assays. Additionally, compounds 11, 23, 30 and 31 displayed potent activity with IC50 values of approximately 0.1 μM, and weak cytotoxicity against mammalian cells. However, in vivo antimalarial activity is not effective, which might be ascribed to the poor solubility of these compounds.
Conclusion: In this study, the phenotypic screen of our compound library resulted in the first report of a 3-cinnamamide framework with antimalarial activity and 40 derivatives were then designed and synthesized. Subsequent structure-activity studies showed that compounds 11, 23, 30 and 31 exhibited the most potent and selective activity against the P. falciparum 3D7 strain with IC50 values around 0.1 μM. Our work herein sets another example of phenotypic screen-based drug discovery, leading to potentially promising candidates of novel antimalarial agents once given further optimization.
中文翻译:

发现 3-肉桂酰氨基-N-取代的苯甲酰胺作为潜在的抗疟药
背景:疟疾是最具破坏性的寄生虫病之一,但抗疟药物的发现仍然极具挑战性。近50年来,开发的新型抗疟药极少,而耐药性的出现却不断出现。
目的:本研究侧重于 3-肉桂酰氨基-N-取代苯甲酰胺的发现、设计、合成和抗疟评估。
方法:在本研究中,我们针对多药敏感性恶性疟原虫 3D7 菌株对我们的化合物库进行了筛选。针对恶性疟原虫 3D7 设计、合成和测试了命中的衍生物,并使用 Peters 的 4 天抑制测试方法评估了最活跃化合物的体内抗疟活性。
结果:检索到的含有3-肉桂基-N-取代苯甲酰胺骨架的命中化合物1首次显示出中等抗疟活性(IC 50 = 1.20 μM)。然后通过简单的四步工作流程合成了一系列衍生物,其中一半在随后的体外试验中表现出比前体 1 稍好的抗疟效果。此外,化合物 11、23、30 和 31 显示出有效的活性,IC 50值约为 0.1 μM,对哺乳动物细胞的细胞毒性较弱。然而,体内抗疟活性无效,这可能是由于这些化合物的溶解性差。
结论:在这项研究中,我们化合物库的表型筛选导致了具有抗疟活性的 3-肉桂酰胺框架的第一份报告,然后设计并合成了 40 种衍生物。随后的结构-活性研究表明,化合物 11、23、30 和 31 对恶性疟原虫 3D7 菌株表现出最有效的选择性活性,IC50 值约为 0.1 μM。我们在此的工作为基于表型筛选的药物发现树立了另一个例子,一旦进一步优化,就会产生潜在的有希望的新型抗疟药候选物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号