Journal of Controlled Release ( IF 10.5 ) Pub Date : 2021-11-16 , DOI: 10.1016/j.jconrel.2021.11.019 Hui Li 1 , Yue Feng 2 , Xiu Zheng 1 , Ming Jia 1 , Zhiqiang Mei 3 , Yao Wang 1 , Zhuo Zhang 1 , Meiling Zhou 4 , Chunhong Li 1
|
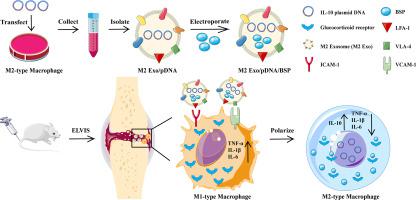
Imbalance between the activities of pro-inflammatory M1 and anti-inflammatory M2 macrophages in rheumatoid arthritis (RA) induces synovial inflammation and autoimmunity, leading to joint damage. Here we encapsulated a plasmid DNA encoding the anti-inflammatory cytokine interleukin-10 (IL-10 pDNA) and the chemotherapeutic drug betamethasone sodium phosphate (BSP) into biomimetic vector M2 exosomes (M2 Exo) derived from M2-type macrophages. We demonstrate that the loaded exosomes target and reduce inflammation for combined therapy against RA. The in vitro efficiency of the M2 Exo/pDNA/BSP co-delivery system was attributed to the synergistic effect of IL-10 pDNA and BSP, which also promoted M1-to-M2 macrophage polarization by reducing the secretion of pro-inflammatory cytokines (IL-1β, TNF-α) and increasing the expression of IL-10 cytokine. In a mouse model of RA, M2 Exo/pDNA/BSP showed good accumulation at inflamed joint sites, high anti-inflammatory activity, and potent therapeutic effect. The delivery system was non-toxic both in vitro and in vivo. Thus, this system may serve as a promising biocompatible drug carrier and anti-inflammatory agent for RA treatment based on M1-to-M2 macrophage re-polarization.
中文翻译:

M2型外泌体纳米粒通过巨噬细胞再极化治疗类风湿关节炎
类风湿关节炎 (RA) 中促炎性 M1 和抗炎性 M2 巨噬细胞的活性失衡会诱发滑膜炎症和自身免疫,从而导致关节损伤。在这里,我们将编码抗炎细胞因子白细胞介素 10 (IL-10 pDNA) 和化疗药物倍他米松磷酸钠 (BSP) 的质粒 DNA 封装到源自 M2 型巨噬细胞的仿生载体 M2 外泌体 (M2 Exo) 中。我们证明加载的外泌体靶向并减少针对 RA 的联合治疗的炎症。体外的M2 Exo/pDNA/BSP 共递送系统的效率归因于 IL-10 pDNA 和 BSP 的协同作用,它还通过减少促炎细胞因子 (IL-1β) 的分泌促进 M1 到 M2 巨噬细胞极化, TNF-α) 和增加 IL-10 细胞因子的表达。在 RA 小鼠模型中,M2 Exo/pDNA/BSP 在发炎的关节部位表现出良好的蓄积、高抗炎活性和有效的治疗效果。该递送系统在体外和体内均无毒。因此,该系统可作为基于 M1 到 M2 巨噬细胞再极化的 RA 治疗的有前途的生物相容性药物载体和抗炎剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号