Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-pot synthesis of new alkyl 1-naphthoates bearing quinoline, pyranone and cyclohexenone moieties via metal-free sequential addition/oxidation reactions
RSC Advances ( IF 3.9 ) Pub Date : 2021-11-16 , DOI: 10.1039/d1ra07092d Seyedeh Hekmat Mousavi 1 , Mohammad Reza Mohammadizadeh 1 , Samira Poorsadeghi 2 , Satoru Arimitsu 2 , Fatemeh Mohammadsaleh 1 , Genta Kojya 3 , Shinichi Gima 3
RSC Advances ( IF 3.9 ) Pub Date : 2021-11-16 , DOI: 10.1039/d1ra07092d Seyedeh Hekmat Mousavi 1 , Mohammad Reza Mohammadizadeh 1 , Samira Poorsadeghi 2 , Satoru Arimitsu 2 , Fatemeh Mohammadsaleh 1 , Genta Kojya 3 , Shinichi Gima 3
Affiliation
|
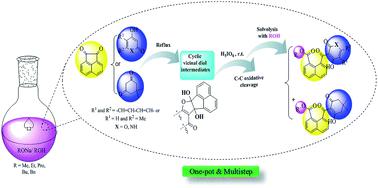
A mild and one-pot synthetic pathway was successfully developed for the synthesis of new naphthoate-based scaffolds containing quinoline, pyranone and cyclohexenone moieties via a multistep reaction between acenaphthoquinone and various 1,3-diketones in the presence of different primary aliphatic and benzylic alcohols. This reaction proceeds via a sequential addition/oxidation mechanistic process including a metal-free addition step of acenaphthoquinone and 1,3-diketones followed by the H5IO6-mediated C–C oxidative cleavage of the corresponding vicinal diols at room temperature. The alcohols play a dual role, as the reaction solvent as well as the nucleophile, to conduct the reaction process toward naphthoate formation. All alkyl naphthoate derivatives prepared in this work are new compounds and were definitively characterized using 1H-NMR, 13C-NMR and HRMS analysis, while X-ray crystallography was carried out for one of the products. The synthesis of a naphthalene-based nucleus attached to heterocyclic moieties is noteworthy to follow in the near future for diverse applications in biology, medicine, metal complex design, and semiconductor and optical materials.
中文翻译:

通过无金属顺序加成/氧化反应一锅法合成含有喹啉、吡喃酮和环己烯酮部分的新型烷基 1-萘甲酸酯
通过苊醌和各种 1,3-二酮在不同的脂肪族伯醇和苯甲醇存在下的多步反应,成功开发了一种温和的一锅法合成途径,用于合成含有喹啉、吡喃酮和环己烯酮部分的新型萘甲酸酯基支架。. 该反应通过顺序加成/氧化机制过程进行,包括苊醌和 1,3-二酮的无金属加成步骤,然后是 H 5 IO 6介导的 C-C 氧化裂解相应的邻位二醇在室温下。醇发挥双重作用,作为反应溶剂和亲核试剂,以进行反应过程以形成萘甲酸盐。在这项工作中制备的所有萘甲酸烷基酯衍生物都是新化合物,并使用1 H-NMR、13 C-NMR 和 HRMS 分析进行了明确表征,同时对其中一种产品进行了 X 射线晶体学。在不久的将来,在生物学、医学、金属配合物设计以及半导体和光学材料中的各种应用中,值得关注的是,与杂环基团相连的萘基核的合成。
更新日期:2021-11-16
中文翻译:

通过无金属顺序加成/氧化反应一锅法合成含有喹啉、吡喃酮和环己烯酮部分的新型烷基 1-萘甲酸酯
通过苊醌和各种 1,3-二酮在不同的脂肪族伯醇和苯甲醇存在下的多步反应,成功开发了一种温和的一锅法合成途径,用于合成含有喹啉、吡喃酮和环己烯酮部分的新型萘甲酸酯基支架。. 该反应通过顺序加成/氧化机制过程进行,包括苊醌和 1,3-二酮的无金属加成步骤,然后是 H 5 IO 6介导的 C-C 氧化裂解相应的邻位二醇在室温下。醇发挥双重作用,作为反应溶剂和亲核试剂,以进行反应过程以形成萘甲酸盐。在这项工作中制备的所有萘甲酸烷基酯衍生物都是新化合物,并使用1 H-NMR、13 C-NMR 和 HRMS 分析进行了明确表征,同时对其中一种产品进行了 X 射线晶体学。在不久的将来,在生物学、医学、金属配合物设计以及半导体和光学材料中的各种应用中,值得关注的是,与杂环基团相连的萘基核的合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号