当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insights into Superior Nitrate Reduction to Ammonia Performance of Copper Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-11-15 , DOI: 10.1021/acscatal.1c03666 Tao Hu 1 , Changhong Wang 1 , Mengting Wang 1 , Chang Ming Li 1, 2 , Chunxian Guo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-11-15 , DOI: 10.1021/acscatal.1c03666 Tao Hu 1 , Changhong Wang 1 , Mengting Wang 1 , Chang Ming Li 1, 2 , Chunxian Guo 1
Affiliation
|
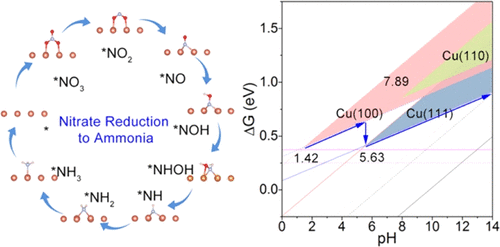
Nitrate reduction to ammonia (NRA) is critical and attractive for environmental remediation and energy conservation. Copper represents one of the most promising non-noble-metal NRA electrocatalysts while its intrinsic catalytic activity of facets and pH influence remain unclear. Using density functional theory calculations, nitrate reduction to ammonia pathways are evaluated on low-index crystal surfaces, Cu(111), Cu(100), and Cu(110), at different pH. Systematic thermodynamic and kinetic analysis indicates that the pathway NO3– → *NO3 → *NO2 → *NO → *NOH → *NHOH → *NH → *NH2 → *NH3 → NH3(g) is the most probable in all pH ranges, ending a long-standing debate on NRA pathways. Both the catalytic deoxygenation and hydrogenation processes in NRA are substantially affected by pH. Thus, the rate-determining steps and overpotentials exhibit pH-dependent characteristics. Besides, it is found that the pH influences the competition between the hydrogen evolution reaction (HER) and NRA. By considering NRA and HER on different surfaces, we found that Cu(100) and Cu(111) contribute most to NRA other than Cu(110). Specifically, in near-neutral and alkaline environments, Cu(111) exhibits the best NO3– to NH3 performance, while Cu(100) is more effective in a strong acidic environment. This result rationalizes recent experimental observations. The NRA activity differences of copper surfaces are attributed to the local coordination environment and electronic states of surface atoms. Thanks to a stereospecific Cu–Cu couple, both strong *NOH adsorption and weak *NH3 adsorption are realized on Cu(111) and Cu(100), facilitating superior NRA.
中文翻译:

铜催化剂对氨性能的优越硝酸盐还原的理论见解
硝酸盐还原成氨 (NRA) 对环境修复和节能至关重要且具有吸引力。铜是最有前途的非贵金属 NRA 电催化剂之一,但其固有的小平面催化活性和 pH 值影响尚不清楚。使用密度泛函理论计算,在不同 pH 值下,在低指数晶体表面 Cu(111)、Cu(100) 和 Cu(110) 上评估硝酸盐还原为氨的途径。系统热力学和动力学分析表明NO 3 – → *NO 3 → *NO 2 → *NO → *NOH → *NHOH → *NH → *NH 2 → *NH 3 → NH 3(g) 在所有 pH 范围内最有可能,结束了关于 NRA 途径的长期争论。NRA 中的催化脱氧和加氢过程都受到 pH 值的显着影响。因此,速率决定步骤和过电位表现出 pH 依赖性特征。此外,还发现pH影响析氢反应(HER)和NRA之间的竞争。通过考虑不同表面上的 NRA 和 HER,我们发现除了 Cu(110) 之外,Cu(100) 和 Cu(111) 对 NRA 的贡献最大。具体而言,在近中性和碱性环境中,Cu(111) 表现出最佳的 NO 3 – NH 3性能,而 Cu(100) 在强酸性环境中更有效。这一结果使最近的实验观察合理化。铜表面的 NRA 活性差异归因于局部配位环境和表面原子的电子状态。由于立体定向的Cu-Cu 对,在Cu(111) 和Cu(100) 上实现了强*NOH 吸附和弱*NH 3吸附,促进了优异的NRA。
更新日期:2021-12-03
中文翻译:

铜催化剂对氨性能的优越硝酸盐还原的理论见解
硝酸盐还原成氨 (NRA) 对环境修复和节能至关重要且具有吸引力。铜是最有前途的非贵金属 NRA 电催化剂之一,但其固有的小平面催化活性和 pH 值影响尚不清楚。使用密度泛函理论计算,在不同 pH 值下,在低指数晶体表面 Cu(111)、Cu(100) 和 Cu(110) 上评估硝酸盐还原为氨的途径。系统热力学和动力学分析表明NO 3 – → *NO 3 → *NO 2 → *NO → *NOH → *NHOH → *NH → *NH 2 → *NH 3 → NH 3(g) 在所有 pH 范围内最有可能,结束了关于 NRA 途径的长期争论。NRA 中的催化脱氧和加氢过程都受到 pH 值的显着影响。因此,速率决定步骤和过电位表现出 pH 依赖性特征。此外,还发现pH影响析氢反应(HER)和NRA之间的竞争。通过考虑不同表面上的 NRA 和 HER,我们发现除了 Cu(110) 之外,Cu(100) 和 Cu(111) 对 NRA 的贡献最大。具体而言,在近中性和碱性环境中,Cu(111) 表现出最佳的 NO 3 – NH 3性能,而 Cu(100) 在强酸性环境中更有效。这一结果使最近的实验观察合理化。铜表面的 NRA 活性差异归因于局部配位环境和表面原子的电子状态。由于立体定向的Cu-Cu 对,在Cu(111) 和Cu(100) 上实现了强*NOH 吸附和弱*NH 3吸附,促进了优异的NRA。































 京公网安备 11010802027423号
京公网安备 11010802027423号