当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of fenchone by broadband rotational spectroscopy
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-08-19 12:30:41 , DOI: 10.1063/1.4961018 Donatella Loru 1 , Miguel A. Bermúdez 1 , M. Eugenia Sanz 1
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-08-19 12:30:41 , DOI: 10.1063/1.4961018 Donatella Loru 1 , Miguel A. Bermúdez 1 , M. Eugenia Sanz 1
Affiliation
|
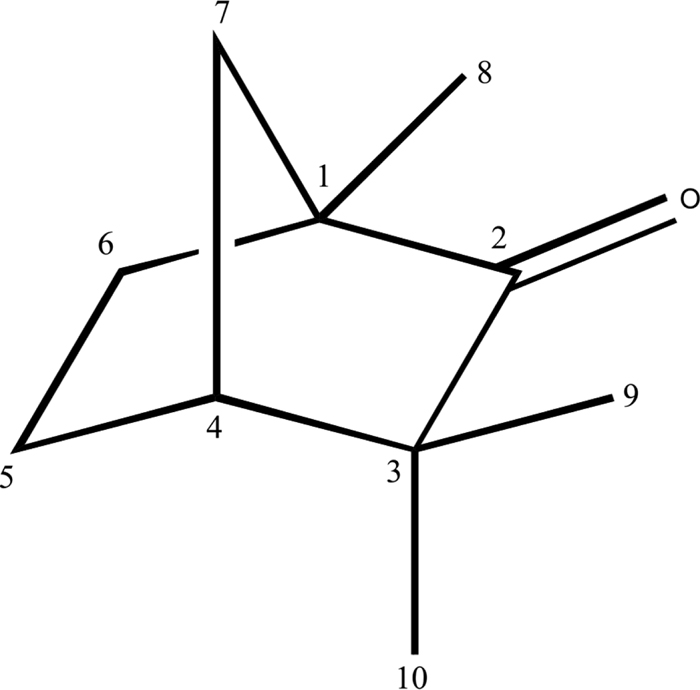
The bicyclic terpenoid fenchone (C10H16O, 1,3,3-trimethylbicyclo[2.2.1]heptan-2-one) has been investigated by chirped pulse Fourier transform microwave spectroscopy in the 2-8 GHz frequency region. The parent species and all heavy atom isotopologues have been observed in their natural abundance. The experimental rotational constants of all isotopic species observed have been determined and used to obtain the substitution (r s) and effective (r 0) structures of fenchone. Calculations at the B3LYP, M06-2X, and MP2 levels of theory with different basis sets were carried out to check their performance against experimental results. The structure of fenchone has been compared with those of norbornane (bicyclo[2.2.1]heptane) and the norbornane derivatives camphor (1,7,7-trimethylbicyclo[2.2.1]heptan-2-one) and camphene (3,3-dimethyl-2-methylenebicyclo[2.2.1]heptane), both with substituents at C2. The structure of fenchone is remarkably similar to those of camphor and camphene. Comparison with camphor allows identification of changes in ∠CCC angles due to the different position of the methyl groups. All norbornane derivatives display similar structural changes with respect to norbornane. These changes mainly affect the bond lengths and angles of the six-membered rings, indicating that the substituent at C2 drives structural adjustments to minimise ring strain after its introduction.
中文翻译:

宽带旋转光谱法测定芬甲酮的结构
已通过chi脉冲傅里叶变换微波光谱法研究了2-8 GHz频率范围内的双环萜烯类琴酮(C 10 H 16 O,1,3,3-三甲基双环[2.2.1]庚-2-酮)。亲本物种和所有重原子的同位素都已观察到其自然丰度。已确定观察到的所有同位素的实验旋转常数,并用于获得替代(r s)和有效替代(r 0)Fenchone的结构。在B3LYP,M06-2X和MP2的理论水平上使用不同的基础集进行了计算,以检查它们相对于实验结果的性能。己烯酮的结构已与降冰片烷(双环[2.2.1]庚烷)和降冰片烷衍生物樟脑(1,7,7-三甲基双环[2.2.1]庚烷-2-一)和樟脑(3,3)的结构进行了比较-二甲基-2-亚甲基双环[2.2.1]庚烷),均在C 2处具有取代基。Fenchone的结构与樟脑和樟脑的结构非常相似。与樟脑的比较可以确定由于甲基位置不同而引起的∠CCC角变化。所有降冰片烷衍生物相对于降冰片烷显示出相似的结构变化。这些变化主要影响六元环的键长和角度,表明在C 2处的取代基驱动结构调整,以最大程度地降低引入环后的环应变。
更新日期:2016-08-20
中文翻译:

宽带旋转光谱法测定芬甲酮的结构
已通过chi脉冲傅里叶变换微波光谱法研究了2-8 GHz频率范围内的双环萜烯类琴酮(C 10 H 16 O,1,3,3-三甲基双环[2.2.1]庚-2-酮)。亲本物种和所有重原子的同位素都已观察到其自然丰度。已确定观察到的所有同位素的实验旋转常数,并用于获得替代(r s)和有效替代(r 0)Fenchone的结构。在B3LYP,M06-2X和MP2的理论水平上使用不同的基础集进行了计算,以检查它们相对于实验结果的性能。己烯酮的结构已与降冰片烷(双环[2.2.1]庚烷)和降冰片烷衍生物樟脑(1,7,7-三甲基双环[2.2.1]庚烷-2-一)和樟脑(3,3)的结构进行了比较-二甲基-2-亚甲基双环[2.2.1]庚烷),均在C 2处具有取代基。Fenchone的结构与樟脑和樟脑的结构非常相似。与樟脑的比较可以确定由于甲基位置不同而引起的∠CCC角变化。所有降冰片烷衍生物相对于降冰片烷显示出相似的结构变化。这些变化主要影响六元环的键长和角度,表明在C 2处的取代基驱动结构调整,以最大程度地降低引入环后的环应变。































 京公网安备 11010802027423号
京公网安备 11010802027423号