当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Construction of 2-Amino-2′-ketonyl Biaryls via Acid-Mediated Ring Opening of 9H-Fluoren-9-ols with Organic Azides
Organic Letters ( IF 4.9 ) Pub Date : 2021-11-15 , DOI: 10.1021/acs.orglett.1c03484 Shan Yang 1 , Biqiong Hong 2 , Jia Feng 1 , Zhenhua Gu 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2021-11-15 , DOI: 10.1021/acs.orglett.1c03484 Shan Yang 1 , Biqiong Hong 2 , Jia Feng 1 , Zhenhua Gu 1, 2
Affiliation
|
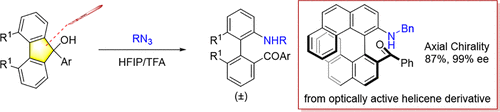
A direct cross-coupling between 9H-fluoren-9-ols and organic azides for the synthesis of steric hindered 2-amino-2′-ketonyl biaryls was reported. The reaction featured an acid-mediated azidation/ring-expansion/hydrolysis cascade, which formally realized the C–N bond coupling reaction via cleavage of a C–C single bond. This method was applicable to chiral helical structure to give bulky axially chiral biaryls with full stereospecificity.
中文翻译:

通过酸介导的 9H-Fluoren-9-ols 与有机叠氮化物的开环构建 2-Amino-2'-ketonyl Biaryls
报道了 9 H -fluoren-9-ols 和有机叠氮化物之间的直接交叉偶联,用于合成空间位阻 2-amino-2'-ketonyl biaryls。该反应具有酸介导的叠氮化/扩环/水解级联反应,通过C-C单键的断裂正式实现了C-N键偶联反应。该方法适用于手性螺旋结构,得到具有完全立体定向的大体积轴向手性联芳基化合物。
更新日期:2021-12-03
中文翻译:

通过酸介导的 9H-Fluoren-9-ols 与有机叠氮化物的开环构建 2-Amino-2'-ketonyl Biaryls
报道了 9 H -fluoren-9-ols 和有机叠氮化物之间的直接交叉偶联,用于合成空间位阻 2-amino-2'-ketonyl biaryls。该反应具有酸介导的叠氮化/扩环/水解级联反应,通过C-C单键的断裂正式实现了C-N键偶联反应。该方法适用于手性螺旋结构,得到具有完全立体定向的大体积轴向手性联芳基化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号