Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2021-11-13 , DOI: 10.1016/j.jcis.2021.11.040 Tzu−Ho Wu, Yu−Ming Li, Kung−Yi Ni, Tzu-Kuan Li, Wei−Sheng Lin
|
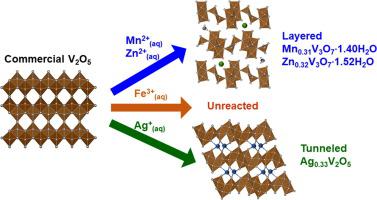
Rechargeable aqueous zinc−ion batteries (RAZIBs) have received increasing attention due to cost−effectiveness and inherent safety. A wide variety of advanced cathode materials have been revealed with promising performance in RAZIBs. However, these materials usually require sophisticated procedures at high temperatures, which greatly limit further practical application. Herein, a chimie douce approach is adopted to prepare vanadium oxides from V2O5 suspension with the addition of various transition metal cations (Mn2+, Zn2+, Ag+, and Fe3+) by simple liquid−solid mixing under ambient conditions. For the cases of Mn2+ and Zn2+, the dissolution−recrystallization process takes place leading to layered Mn0.31V3O7·1.40H2O (MnVO) and Zn0.32V3O7·1.52H2O (ZnVO). The use of Ag+ forms tunneled Ag0.33V2O5 (AgVO), and the present of Fe3+ stays mainly unreacted V2O5. The underlying reaction chemistries are proposed, for which the pH values of precursor solutions are found to be a key factor. Among the prepared materials, layered vanadium oxides exhibit promising battery performance. Particularly, MnVO delivers 340 and 217 mAh g−1 at 1 and 8 A g−1, respectively. A specific capacity of 164 mAh g−1 can be retained after 500 cycles at 1 A g−1. By constrast, AgVO and FeVO demonstrate inferior performance with retaining only 89 and 20 mAh g−1 after 500 cycles.
中文翻译:

Chimie Douce 反应获得的氧化钒:过渡金属种类对锌离子电池晶体结构和电化学行为的影响
由于成本效益和固有的安全性,可充电水性锌离子电池(RAZIB)受到越来越多的关注。各种先进的正极材料已被揭示在 RAZIBs 中具有良好的性能。然而,这些材料通常需要在高温下进行复杂的程序,这极大地限制了进一步的实际应用。在本文中,通过简单的液-固混合,通过加入各种过渡金属阳离子(Mn 2+、Zn 2+、Ag +和Fe 3+),采用chimie douce 方法从V 2 O 5悬浮液中制备钒氧化物。环境条件。对于Mn 2+和Zn 2+ 的情况,溶解-再结晶过程发生导致层状Mn 0.31 V 3 O 7 ·1.40H 2 O (MnVO) 和Zn 0.32 V 3 O 7 ·1.52H 2 O (ZnVO)。Ag +的使用形成隧道 Ag 0.33 V 2 O 5 (AgVO),Fe 3+的存在主要是未反应的 V 2 O 5. 提出了潜在的反应化学,其中前体溶液的 pH 值被认为是一个关键因素。在制备的材料中,层状钒氧化物表现出良好的电池性能。特别是,MnVO在 1 和 8 A g -1时分别提供 340 和 217 mAh g -1。在 1 A g -1 下循环 500 次后可保持164 mAh g -1 的比容量。相比之下,AgVO 和 FeVO 表现出较差的性能,在 500 次循环后仅保留 89 和 20 mAh g -1。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号