当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Insight into the β-Sheet Twist and Related Morphology of Self-Assembled Peptide Amphiphile Ribbons
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-11-11 , DOI: 10.1021/acs.jpclett.1c03243 Qinsi Xiong 1 , Samuel I Stupp 2, 3, 4, 5 , George C Schatz 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-11-11 , DOI: 10.1021/acs.jpclett.1c03243 Qinsi Xiong 1 , Samuel I Stupp 2, 3, 4, 5 , George C Schatz 1
Affiliation
|
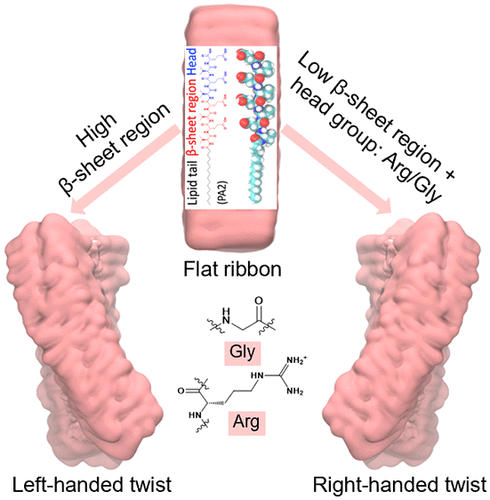
Self-assembly of high-aspect-ratio filaments containing β-sheets has attracted much attention due to potential use in bioengineering and biomedicine. However, precisely predicting the assembled morphologies remains a grand challenge because of insufficient understanding of the self-assembly process. We employed an atomistic model to study the self-assembly of peptide amphiphiles (PAs) containing valine–glutamic acid (VE) dimeric repeats. By changing of the sequence length, the assembly morphology changes from flat ribbon to left-handed twisted ribbon, implying a relationship between β-sheet twist and strength of interstrand hydrogen bonds. The calculations are used to quantify this relationship including both magnitude and sign of the ribbon twist angle. Interestingly, a change in chirality is observed when we introduce the RGD epitope into the C-terminal of VE repeats, suggesting arginine and glycine’s role in suppressing right-handed β-sheet formation. This study provides insight into the relationship between β-sheet twist and self-assembled nanostructures including a possible design rule for PA self-assembly.
中文翻译:

对自组装肽两亲带的 β-折叠和相关形态的分子洞察
含有β-折叠的高纵横比长丝的自组装由于在生物工程和生物医学中的潜在用途而备受关注。然而,由于对自组装过程的理解不足,精确预测组装的形态仍然是一个巨大的挑战。我们采用原子模型来研究含有缬氨酸 - 谷氨酸(VE)二聚重复序列的肽两亲物(PAs)的自组装。通过改变序列长度,组装形态从扁平带状变为左旋扭曲带状,暗示β-折叠扭曲与链间氢键强度之间存在关系。计算用于量化这种关系,包括带扭曲角的大小和符号。有趣的是,当我们将 RGD 表位引入 VE 重复序列的 C 末端时,观察到手性的变化,这表明精氨酸和甘氨酸在抑制右手 β-折叠形成中的作用。这项研究提供了对 β-折叠扭曲和自组装纳米结构之间关系的深入了解,包括 PA 自组装的可能设计规则。
更新日期:2021-11-25
中文翻译:

对自组装肽两亲带的 β-折叠和相关形态的分子洞察
含有β-折叠的高纵横比长丝的自组装由于在生物工程和生物医学中的潜在用途而备受关注。然而,由于对自组装过程的理解不足,精确预测组装的形态仍然是一个巨大的挑战。我们采用原子模型来研究含有缬氨酸 - 谷氨酸(VE)二聚重复序列的肽两亲物(PAs)的自组装。通过改变序列长度,组装形态从扁平带状变为左旋扭曲带状,暗示β-折叠扭曲与链间氢键强度之间存在关系。计算用于量化这种关系,包括带扭曲角的大小和符号。有趣的是,当我们将 RGD 表位引入 VE 重复序列的 C 末端时,观察到手性的变化,这表明精氨酸和甘氨酸在抑制右手 β-折叠形成中的作用。这项研究提供了对 β-折叠扭曲和自组装纳米结构之间关系的深入了解,包括 PA 自组装的可能设计规则。































 京公网安备 11010802027423号
京公网安备 11010802027423号