当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gram-Scale Synthesis of Bufospirostenin A by a Biomimetic Skeletal Rearrangement Approach
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-11 , DOI: 10.1021/jacs.1c10067 Yu Wang 1 , Hailong Tian 1 , Jinghan Gui 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-11 , DOI: 10.1021/jacs.1c10067 Yu Wang 1 , Hailong Tian 1 , Jinghan Gui 1
Affiliation
|
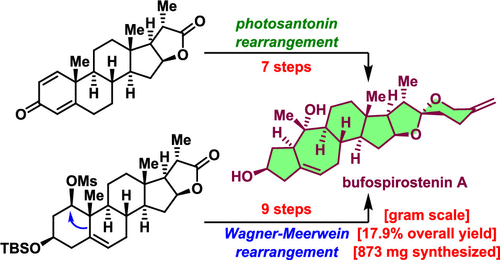
Bufospirostenin A, which was the first spirostanol to be isolated from an animal, possesses an unprecedented 5/7/6/5/5/6 hexacyclic framework. Herein, we report two biomimetic syntheses of this natural product in just seven or nine steps from a readily available steroidal lactone. Key features of the syntheses include a photosantonin rearrangement and a Wagner–Meerwein rearrangement for rapid construction of the rearranged A/B ring system, as well as a cobalt-mediated olefin hydroselenylation and a selenide E2 reaction to accomplish a challenging olefin transposition. Our syntheses provide experimental support for the biogenetic pathway to 5(10→1)abeo-steroids that we have proposed.
中文翻译:

通过仿生骨骼重排方法合成 Bufospirostenin A 的革兰氏规模
Bufospirostenin A 是第一个从动物中分离出来的螺甾烷醇,它拥有前所未有的 5/7/6/5/5/6 六环骨架。在这里,我们报告了这种天然产物的两种仿生合成,只需七到九个步骤,就可以从现成的甾体内酯中获得。合成的关键特征包括用于快速构建重排的 A/B 环系统的 photosantonin 重排和 Wagner-Meerwein 重排,以及用于完成具有挑战性的烯烃转位的钴介导的烯烃氢化硒化和硒化物 E2 反应。我们的合成为我们提出的 5(10→1) abeo-类固醇的生物遗传途径提供了实验支持。
更新日期:2021-11-24
中文翻译:

通过仿生骨骼重排方法合成 Bufospirostenin A 的革兰氏规模
Bufospirostenin A 是第一个从动物中分离出来的螺甾烷醇,它拥有前所未有的 5/7/6/5/5/6 六环骨架。在这里,我们报告了这种天然产物的两种仿生合成,只需七到九个步骤,就可以从现成的甾体内酯中获得。合成的关键特征包括用于快速构建重排的 A/B 环系统的 photosantonin 重排和 Wagner-Meerwein 重排,以及用于完成具有挑战性的烯烃转位的钴介导的烯烃氢化硒化和硒化物 E2 反应。我们的合成为我们提出的 5(10→1) abeo-类固醇的生物遗传途径提供了实验支持。






























 京公网安备 11010802027423号
京公网安备 11010802027423号