当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
p-Terphenyls as Anti-HSV-1/2 Agents from a Deep-Sea-Derived Penicillium sp.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-11-12 , DOI: 10.1021/acs.jnatprod.1c00400
Weihao Chen 1, 2 , Jiawen Zhang 3 , Xin Qi 1 , Kai Zhao 1 , Xiaoyan Pang 1 , Xiuping Lin 1, 4, 5 , Shengrong Liao 1, 4, 5 , Bin Yang 1, 4, 5 , Xuefeng Zhou 1, 4, 5 , Shuwen Liu 3 , Junfeng Wang 1, 4, 5 , Xingang Yao 3 , Yonghong Liu 1, 2, 4, 5
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-11-12 , DOI: 10.1021/acs.jnatprod.1c00400
Weihao Chen 1, 2 , Jiawen Zhang 3 , Xin Qi 1 , Kai Zhao 1 , Xiaoyan Pang 1 , Xiuping Lin 1, 4, 5 , Shengrong Liao 1, 4, 5 , Bin Yang 1, 4, 5 , Xuefeng Zhou 1, 4, 5 , Shuwen Liu 3 , Junfeng Wang 1, 4, 5 , Xingang Yao 3 , Yonghong Liu 1, 2, 4, 5
Affiliation
|
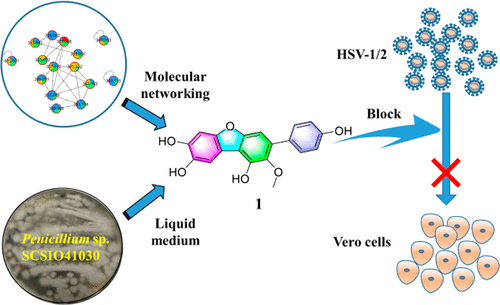
Guided by Global Natural Products Social molecular networking, two p-terphenyl derivatives and one 4,5-diphenyl-2-pyrone analogue, peniterphenyls A–C (1–3), together with five known p-terphenyl derivatives (4–8) and sulochrin (9), were obtained from a deep-sea-derived Penicillium sp. SCSIO41030. Their structures were elucidated using extensive NMR spectroscopic and HRESIMS data and by comparing the information with literature data. Peniterphenyl B (2) represented the first reported natural product possessing a 4,5-diphenyl-substituted 2-pyrone derivative. The p-terphenyl derivatives displayed inhibitory activities against HSV-1/2 with EC50 values ranging from 1.4 ± 0.6 to 9.3 ± 3.7 μM in Vero cells, which showed that they possessed antiviral activities with low cytotoxicity, superior to the current clinical drug acyclovir (EC50 3.6 ± 0.7 μM). Peniterphenyl A (1) inhibited HSV-1/2 virus entry into cells and may block HSV-1/2 infection through direct interaction with virus envelope glycoprotein D to interfere with virus adsorption and membrane fusion, and thus differs from the nucleoside analogues such as acyclovir. Our study indicated peniterphenyl A (1) could be a promising lead compound against HSV-1/2.
中文翻译:

对三联苯作为来自深海衍生的青霉属的抗 HSV-1/2 药剂。
在全球天然产物社会分子网络的指导下,两种对三联苯衍生物和一种 4,5-二苯基-2-吡喃酮类似物,对三联苯 A-C ( 1 - 3 ),以及五种已知的对三联苯衍生物 ( 4 - 8 )和 sulochrin ( 9 ),从深海衍生的青霉获得。SCSIO41030。使用广泛的 NMR 光谱和 HRESIMS 数据并通过将信息与文献数据进行比较,阐明了它们的结构。Peniterphenyl B ( 2 ) 代表了第一个报道的具有4,5-二苯基取代的2-吡喃酮衍生物的天然产物。p _-三联苯衍生物对 HSV-1/2 具有抑制活性,在 Vero 细胞中的 EC 50值范围为 1.4 ± 0.6 至 9.3 ± 3.7 μM,表明它们具有低细胞毒性的抗病毒活性,优于目前的临床药物阿昔洛韦(EC 50 3.6 ± 0.7 微米)。Peniterphenyl A ( 1 ) 抑制 HSV-1/2 病毒进入细胞,可能通过与病毒包膜糖蛋白 D 直接相互作用,干扰病毒吸附和膜融合,从而阻断 HSV-1/2 感染,因此不同于核苷类似物,如阿昔洛韦。我们的研究表明 Peniterphenyl A ( 1 ) 可能是一种有前途的抗 HSV-1/2 的先导化合物。
更新日期:2021-11-26
中文翻译:

对三联苯作为来自深海衍生的青霉属的抗 HSV-1/2 药剂。
在全球天然产物社会分子网络的指导下,两种对三联苯衍生物和一种 4,5-二苯基-2-吡喃酮类似物,对三联苯 A-C ( 1 - 3 ),以及五种已知的对三联苯衍生物 ( 4 - 8 )和 sulochrin ( 9 ),从深海衍生的青霉获得。SCSIO41030。使用广泛的 NMR 光谱和 HRESIMS 数据并通过将信息与文献数据进行比较,阐明了它们的结构。Peniterphenyl B ( 2 ) 代表了第一个报道的具有4,5-二苯基取代的2-吡喃酮衍生物的天然产物。p _-三联苯衍生物对 HSV-1/2 具有抑制活性,在 Vero 细胞中的 EC 50值范围为 1.4 ± 0.6 至 9.3 ± 3.7 μM,表明它们具有低细胞毒性的抗病毒活性,优于目前的临床药物阿昔洛韦(EC 50 3.6 ± 0.7 微米)。Peniterphenyl A ( 1 ) 抑制 HSV-1/2 病毒进入细胞,可能通过与病毒包膜糖蛋白 D 直接相互作用,干扰病毒吸附和膜融合,从而阻断 HSV-1/2 感染,因此不同于核苷类似物,如阿昔洛韦。我们的研究表明 Peniterphenyl A ( 1 ) 可能是一种有前途的抗 HSV-1/2 的先导化合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号