European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-11-12 , DOI: 10.1016/j.ejmech.2021.113987 Liyu Zhao 1 , Yin Sun 1 , Wenbo Yin 1 , Linfeng Tian 1 , Nannan Sun 1 , Yang Zheng 1 , Chu Zhang 1 , Shizhen Zhao 2 , Xin Su 3 , Dongmei Zhao 1 , Maosheng Cheng 1
|
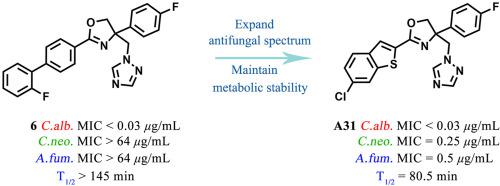
To discover antifungal compounds with broad-spectrum and stable metabolism, a series of 2-(benzo[b]thiophen-2-yl)-4-phenyl-4,5-dihydrooxazole derivatives was designed and synthesized. Compounds A30-A34 exhibited excellent broad-spectrum antifungal activity against Candida albicans with MIC values in the range of 0.03–0.5 μg/mL, and against Cryptococcus neoformans and Aspergillus fumigatus with MIC values in the range of 0.25–2 μg/mL. In addition, compounds A31 and A33 showed high metabolic stability in human liver microsomes in vitro, with the half-life of 80.5 min and 69.4 min, respectively. Moreover, compounds A31 and A33 showed weak or almost no inhibitory effect on the CYP3A4 and CYP2D6. The pharmacokinetic evaluation in SD rats showed that compound A31 had suitable pharmacokinetic properties and was worthy of further study.
中文翻译:

2-(苯并[b]噻吩-2-基)-4-苯基-4,5-二氢恶唑衍生物作为广谱抗真菌剂的设计、合成和生物活性评价
为发现广谱、稳定代谢的抗真菌化合物,设计合成了一系列2-(苯并[ b ]噻吩-2-基)-4-苯基-4,5-二氢恶唑衍生物。化合物A30-A34对白色念珠菌具有优异的广谱抗真菌活性,MIC 值在 0.03-0.5 μg /mL 范围内,对新型隐球菌和烟曲霉的 MIC 值在 0.25-2 μg / mL范围内. 此外,化合物A31和A33在体外人肝微粒体中表现出高代谢稳定性,半衰期分别为 80.5 分钟和 69.4 分钟。此外,化合物A31和A33对 CYP3A4 和 CYP2D6 显示出微弱的抑制作用或几乎没有抑制作用。SD大鼠的药代动力学评价表明,化合物A31具有适宜的药代动力学性质,值得进一步研究。































 京公网安备 11010802027423号
京公网安备 11010802027423号