Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-11-11 , DOI: 10.1016/j.bioorg.2021.105471 Shi Ding 1 , Xiaoyong Dong 2 , Ziye Gao 2 , Xiangshan Zheng 2 , Jingchao Ji 2 , Mingjuan Zhang 2 , Fang Liu 2 , Shuang Wu 2 , Min Li 2 , Wenshan Song 2 , Jiwei Shen 1 , Wenwen Duan 3 , Ju Liu 1 , Ye Chen 1
|
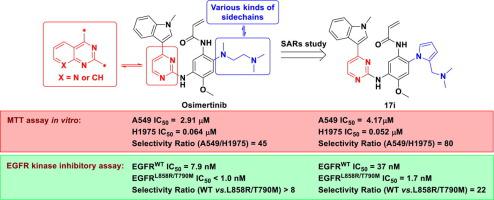
On the basis of N-(3-amino-4-methoxyphenyl)acrylamide scaffold, a series of novel compounds containing 3-substitutional-1-methyl-1H-indole, 2-substitutional pyrrole or thiophene moieties were synthesized and their in vitro antiproliferation activities against A549 and H1975 cell lines were evaluated. The results indicated that most of the compounds showed moderate to excellent antitumor activities. Especially, compounds 9a (A549 IC50 = 1.96 μM, H1975 IC50 = 0.095 μM), 17i (A549 IC50 = 4.17 μM, H1975 IC50 = 0.052 μM), 17j (A549 IC50 = 1.67 μM, H1975 IC50 = 0.061 μM) exhibited comparable antitumor activities and selectivity ratios compared to the positive control osimertinib (A549 IC50 = 2.91 μM, H1975 IC50 = 0.064 μM). In vitro inhibitory activities against EGFR kinases containing different mutations were also tested. Compound 17i showed remarkable inhibitory activity (with IC50 value of 1.7 nM) to EGFRL858R/T790M kinase and selectivity (22-folds compared to EGFRWT kinase). Furthermore, acridine orange/ethidium bromide (AO/EB) staining assay, cell apoptosis assay, cell cycle distribution assay and wound-healing assay of the compounds 9a and 17i were performed on H1975 cell line. The results showed dose-dependent activities of the induction of apoptosis, G0/G1-phase arrestation and inhibition of migration, which were similar to the positive control osimertinib. Additionally, molecular docking analysis was performed to seek the possible binding mode between the selected compounds (9a, 17i-17j) and EGFRL858R/T790M kinase. The results demonstrated that compound 17i is a promising candidate and worth further study.
中文翻译:

新型 N-(3-氨基-4-甲氧基苯基)丙烯酰胺衍生物作为选择性 EGFRL858R/T790M 激酶抑制剂的设计、合成和生物学评价
在N- (3-氨基-4-甲氧基苯基)丙烯酰胺支架的基础上,合成了一系列含有3-取代-1-甲基-1H-吲哚、2-取代吡咯或噻吩部分的新型化合物及其体外抗增殖作用评估了针对 A549 和 H1975 细胞系的活性。结果表明,大多数化合物显示出中等至优异的抗肿瘤活性。特别是化合物9a (A549 IC 50 = 1.96 μM, H1975 IC 50 = 0.095 μM), 17i (A549 IC 50 = 4.17 μM, H1975 IC 50 = 0.052 μM), 17j (A549 IC 50 = 1.67 μM, H1975 IC 50= 0.061 μM)与阳性对照奥希替尼(A549 IC 50 = 2.91 μM,H1975 IC 50 = 0.064 μM) 相比,表现出相当的抗肿瘤活性和选择性比。还测试了针对含有不同突变的EGFR激酶的体外抑制活性。化合物17i对 EGFR L858R/T790M激酶显示出显着的抑制活性(IC 50值为 1.7 nM)和选择性(与 EGFR WT激酶相比为 22 倍)。此外,化合物9a和17i的吖啶橙/溴化乙锭 (AO/EB) 染色测定、细胞凋亡测定、细胞周期分布测定和伤口愈合测定在 H1975 细胞系上进行。结果显示细胞凋亡诱导、G0/G1期阻滞和迁移抑制的剂量依赖性活性,与阳性对照奥希替尼相似。此外,进行分子对接分析以寻找所选化合物(9a、17i - 17j)与EGFR L858R/T790M激酶之间可能的结合模式。结果表明,化合物17i是一种很有前途的候选物,值得进一步研究。































 京公网安备 11010802027423号
京公网安备 11010802027423号