Redox Biology ( IF 10.7 ) Pub Date : 2021-11-11 , DOI: 10.1016/j.redox.2021.102184 Pierre Sabatier 1 , Christian M Beusch 1 , Radosveta Gencheva 2 , Qing Cheng 2 , Roman Zubarev 3 , Elias S J Arnér 4
|
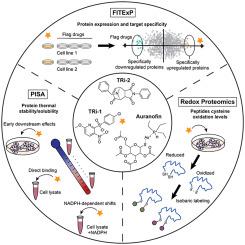
Anticancer drugs that target cellular antioxidant systems have recently attracted much attention. Auranofin (AF) is currently evaluated in several clinical trials as an anticancer agent that targets the cytosolic and mitochondrial forms of the selenoprotein thioredoxin reductase, TXNRD1 and TXNRD2. Recently, two novel TXNRD1 inhibitors (TRi-1 and TRi-2) have been developed that showed anticancer efficacy comparable to AF, but with lower mitochondrial toxicity. However, the cellular action mechanisms of these drugs have not yet been thoroughly studied. Here we used several proteomics approaches to determine the effects of AF, TRi-1 and TRi-2 when used at IC50 concentrations with the mouse B16 melanoma and LLC lung adenocarcinoma cells, as these are often used for preclinical mouse models in evaluation of anticancer drugs. The results demonstrate that TRi-1 and TRi-2 are more specific TXNRD1 inhibitors than AF and reveal additional AF-specific effects on the cellular proteome. Interestingly, AF triggered stronger Nrf2-driven antioxidant responses than the other two compounds. Furthermore, AF affected several additional proteins, including GSK3A, GSK3B, MCMBP and EEFSEC, implicating additional effects on glycogen metabolism, cellular differentiation, inflammatory pathways, DNA replication and selenoprotein synthesis processes. Our proteomics data provide a resource for researchers interested in the multidimensional analysis of proteome changes associated with oxidative stress in general, and the effects of TXNRD1 inhibitors and AF protein targets in particular.
中文翻译:

综合化学蛋白质组学分析表明,新的 TRi-1 和 TRi-2 化合物是比金诺芬更具特异性的硫氧还蛋白还原酶 1 抑制剂
针对细胞抗氧化系统的抗癌药物最近引起了广泛关注。目前,金诺芬 (AF) 作为一种抗癌药物在多项临床试验中得到评估,其靶向胞质和线粒体形式的硒蛋白硫氧还蛋白还原酶 TXNRD1 和 TXNRD2。最近,开发了两种新型 TXNRD1 抑制剂(TRi-1 和 TRi-2),其抗癌功效与 AF 相当,但线粒体毒性较低。然而,这些药物的细胞作用机制尚未得到彻底研究。在这里,我们使用几种蛋白质组学方法来确定 AF、TRi-1 和 TRi-2 在 IC50 浓度下对小鼠 B16 黑色素瘤和 LLC 肺腺癌细胞的影响,因为这些细胞通常用于临床前小鼠模型来评估抗癌药物。结果表明,TRi-1 和 TRi-2 是比 AF 更具特异性的 TXNRD1 抑制剂,并揭示了对细胞蛋白质组的额外 AF 特异性作用。有趣的是,AF 比其他两种化合物引发了更强的 Nrf2 驱动的抗氧化反应。此外,AF 还影响其他几种蛋白质,包括 GSK3A、GSK3B、MCMBP 和 EEFSEC,这意味着对糖原代谢、细胞分化、炎症途径、DNA 复制和硒蛋白合成过程产生额外影响。我们的蛋白质组学数据为对与氧化应激相关的蛋白质组变化的多维分析感兴趣的研究人员提供了资源,特别是 TXNRD1 抑制剂和 AF 蛋白靶点的影响。






























 京公网安备 11010802027423号
京公网安备 11010802027423号