当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurement and Correlation of the Solubility of 4-Biphenylcarboxylic Acid in Nine Pure and Binary Mixed Organic Solvents from T = 293.15 to 328.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-11-09 , DOI: 10.1021/acs.jced.1c00596 Yi Li 1 , Yanqi Wang 1 , Baifeng Hu 1 , Zhengxu Li 1 , Weijian Nie 1 , Qunsheng Li 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-11-09 , DOI: 10.1021/acs.jced.1c00596 Yi Li 1 , Yanqi Wang 1 , Baifeng Hu 1 , Zhengxu Li 1 , Weijian Nie 1 , Qunsheng Li 1
Affiliation
|
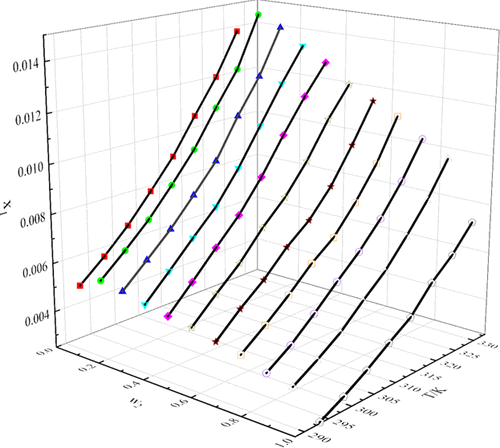
In this paper, we chose gravimetric analysis to measure the solubility of 4-biphenylcarboxylic acid in nine pure and binary mixed organic solvents from T = 293.15 to 328.15 K. It is demonstrated that with the increase of temperature, the solubility of 4-biphenylcarboxylic acid in each of the solvents increases. Meanwhile, the experimental data are fitted with five classical models: Apelblat, van’t Hoff, λh, nonrandom two-liquid (NRTL), and UNIQUAC. The maximum relative average deviations in pure solvents and binary mixed solvents are 0.935 and 1.563%. The fitted result meets the requirements. In addition, this paper also studies the thermodynamic parameters of 4-biphenylcarboxylic acid in the dissolution process and calculates the mixing Gibbs free energy, mixing enthalpy, and mixing entropy during the dissolution process. All of the mixing Gibbs free energy values are less than 0, which shows that the dissolution process is spontaneous.
中文翻译:

在 T = 293.15 至 328.15 K 的九种纯二元混合有机溶剂中 4-联苯甲酸溶解度的测量和相关性
在本文中,我们选择重量分析来测量 4-联苯甲酸在T = 293.15 至 328.15 K 的九种纯二元混合有机溶剂中的溶解度。 结果表明,随着温度的升高,4-联苯甲酸的溶解度在每种溶剂中增加。同时,实验数据拟合了五个经典模型:Apelblat、van't Hoff、λ h、非随机二液 (NRTL) 和 UNIQUAC。纯溶剂和二元混合溶剂的最大相对平均偏差分别为 0.935 和 1.563%。拟合结果满足要求。此外,本文还研究了4-联苯羧酸在溶解过程中的热力学参数,计算了溶解过程中的混合吉布斯自由能、混合焓和混合熵。所有的混合吉布斯自由能值都小于 0,这表明溶解过程是自发的。
更新日期:2021-12-09
中文翻译:

在 T = 293.15 至 328.15 K 的九种纯二元混合有机溶剂中 4-联苯甲酸溶解度的测量和相关性
在本文中,我们选择重量分析来测量 4-联苯甲酸在T = 293.15 至 328.15 K 的九种纯二元混合有机溶剂中的溶解度。 结果表明,随着温度的升高,4-联苯甲酸的溶解度在每种溶剂中增加。同时,实验数据拟合了五个经典模型:Apelblat、van't Hoff、λ h、非随机二液 (NRTL) 和 UNIQUAC。纯溶剂和二元混合溶剂的最大相对平均偏差分别为 0.935 和 1.563%。拟合结果满足要求。此外,本文还研究了4-联苯羧酸在溶解过程中的热力学参数,计算了溶解过程中的混合吉布斯自由能、混合焓和混合熵。所有的混合吉布斯自由能值都小于 0,这表明溶解过程是自发的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号