Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2021-11-10 , DOI: 10.1016/j.cclet.2021.11.009 Yating Liu 1 , Luoyu Wang 1 , Ling-Hui Zeng 2 , Yun Zhao 1 , Tonghao Zhu 1 , Jie Wu 1, 3, 4
|
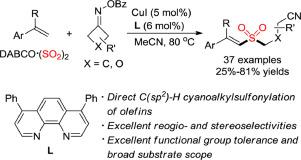
A copper-catalyzed three-component reaction of alkenes, cycloketone oximes and DABCO·(SO2)2 is developed, which provides a convenient route for the synthesis of diverse (E)-cyanoalkylsulfonyl alkenes in moderate to good yields with excellent regio- and stereoselectivity. A broad substrate scope with excellent functional group tolerance is observed. A plausible radical pathway is proposed, which involves copper-catalyzed ring-opening C C bond cleavage of O-acyl oxime and insertion of sulfur dioxide. During the reaction process, cyanoalkyl radical and cyanoalkylsulfonyl radical are the key intermediates.
C bond cleavage of O-acyl oxime and insertion of sulfur dioxide. During the reaction process, cyanoalkyl radical and cyanoalkylsulfonyl radical are the key intermediates.
中文翻译:

铜催化烯烃、环酮肟和 DABCO·(SO2)2 的三组分反应:直接 C(sp2)-H 氰基烷基磺酰化
开发了一种铜催化烯烃、环酮肟和 DABCO·(SO 2 ) 2的三组分反应,为以中等至高产率合成多种 ( E )-氰基烷基磺酰基烯烃提供了一条便捷的途径,具有优异的区域和立体选择性。观察到具有优异官能团耐受性的广泛底物范围。提出了一种似是而非的自由基途径,其涉及铜催化的O- 酰基肟的开环 C C 键断裂和二氧化硫的插入。在反应过程中,氰基烷基自由基和氰基烷基磺酰基自由基是关键的中间体。
酰基肟的开环 C C 键断裂和二氧化硫的插入。在反应过程中,氰基烷基自由基和氰基烷基磺酰基自由基是关键的中间体。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号