当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Preliminary Biological Assessment of Carborane-Loaded Theranostic Nanoparticles to Target Prostate-Specific Membrane Antigen
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-11-09 , DOI: 10.1021/acsami.1c16383
Niranjan Meher 1 , Kyounghee Seo 2 , Sinan Wang 1 , Anil P Bidkar 1 , Miko Fogarty 2 , Suchi Dhrona 1 , Xiao Huang 3 , Ryan Tang 1 , Charles Blaha 3 , Michael J Evans 1, 4, 5 , David R Raleigh 2, 6 , Young-Wook Jun 4, 5, 7 , Henry F VanBrocklin 1, 4 , Tejal A Desai 3 , David M Wilson 1, 4 , Tomoko Ozawa 2 , Robert R Flavell 1, 4, 5
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-11-09 , DOI: 10.1021/acsami.1c16383
Niranjan Meher 1 , Kyounghee Seo 2 , Sinan Wang 1 , Anil P Bidkar 1 , Miko Fogarty 2 , Suchi Dhrona 1 , Xiao Huang 3 , Ryan Tang 1 , Charles Blaha 3 , Michael J Evans 1, 4, 5 , David R Raleigh 2, 6 , Young-Wook Jun 4, 5, 7 , Henry F VanBrocklin 1, 4 , Tejal A Desai 3 , David M Wilson 1, 4 , Tomoko Ozawa 2 , Robert R Flavell 1, 4, 5
Affiliation
|
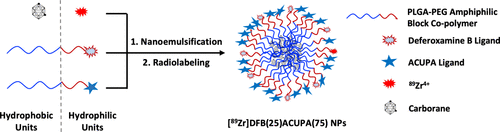
Boron neutron capture therapy (BNCT) is an encouraging therapeutic modality for cancer treatment. Prostate-specific membrane antigen (PSMA) is a cell membrane protein that is abundantly overexpressed in prostate cancer and can be targeted with radioligand therapies to stimulate clinical responses in patients. In principle, a spatially targeted neutron beam together with specifically targeted PSMA ligands could enable prostate cancer-targeted BNCT. Thus, we developed and tested PSMA-targeted poly(lactide-co-glycolide)-block-poly(ethylene glycol) (PLGA-b-PEG) nanoparticles (NPs) loaded with carborane and tethered to the radiometal chelator deferoxamine B (DFB) for simultaneous positron emission tomography (PET) imaging and selective delivery of boron to prostate cancer. Monomeric PLGA-b-PEGs were covalently functionalized with either DFB or the PSMA ligand ACUPA. Different nanoparticle formulations were generated by nanoemulsification of the corresponding unmodified and DFB- or ACUPA-modified monomers in varying percent fractions. The nanoparticles were efficiently labeled with 89Zr and were subjected to in vitro and in vivo evaluation. The optimized DFB(25)ACUPA(75) NPs exhibited strong in vitro binding to PSMA in direct binding and competition radioligand binding assays in PSMA(+) PC3-Pip cells. [89Zr]DFB(25) NPs and [89Zr]DFB(25)ACUPA(75) NPs were injected to mice with bilateral PSMA(−) PC3-Flu and PSMA(+) PC3-Pip dual xenografts. The NPs demonstrated twofold superior accumulation in PC3-Pip tumors to that of PC3-Flu tumors with a tumor/blood ratio of 25; however, no substantial effect of the ACUPA ligands was detected. Moreover, fast release of carborane from the NPs was observed, resulting in a low boron delivery to tumors in vivo. In summary, these data demonstrate the synthesis, characterization, and initial biological assessment of PSMA-targeted, carborane-loaded PLGA-b-PEG nanoparticles and establish the foundation for future efforts to enable their best use in vivo.
中文翻译:

负载碳硼烷的治疗诊断纳米颗粒靶向前列腺特异性膜抗原的合成和初步生物学评估
硼中子俘获疗法 (BNCT) 是一种令人鼓舞的癌症治疗方法。前列腺特异性膜抗原 (PSMA) 是一种细胞膜蛋白,在前列腺癌中大量过度表达,可以用放射配体疗法靶向刺激患者的临床反应。原则上,空间靶向中子束与特异性靶向 PSMA 配体一起可以实现前列腺癌靶向 BNCT。因此,我们开发并测试了 PSMA 靶向的聚(丙交酯-共-乙交酯)-嵌段-聚(乙二醇)(PLGA- b -PEG)纳米粒子(NPs),负载有碳硼烷并与放射性金属螯合剂去铁胺 B(DFB)相连用于同时正电子发射断层扫描 (PET) 成像和选择性地将硼输送到前列腺癌。单体PLGA-b -PEG 与 DFB 或 PSMA 配体 ACUPA 共价官能化。不同的纳米颗粒制剂通过相应的未改性和 DFB 或 ACUPA 改性单体以不同的百分比分数进行纳米乳化而产生。纳米粒子被89 Zr有效标记,并进行了体外和体内评估。优化的DFB(25)ACUPA(75) NPs 在PSMA(+) PC3-Pip 细胞中的直接结合和竞争放射性配体结合试验中表现出与PSMA 的强体外结合。[ 89 Zr]DFB(25) NPs 和 [ 89Zr]DFB(25)ACUPA(75) NPs 被注射到具有双侧 PSMA(-) PC3-Flu 和 PSMA(+) PC3-Pip 双异种移植物的小鼠体内。NPs 在 PC3-Pip 肿瘤中的积累是 PC3-Flu 肿瘤的两倍,肿瘤/血液比为 25;然而,没有检测到 ACUPA 配体的实质性影响。此外,观察到碳硼烷从 NP 中快速释放,导致体内向肿瘤的低硼输送。总之,这些数据证明了 PSMA 靶向、负载碳硼烷的 PLGA- b - PEG 纳米颗粒的合成、表征和初步生物学评估,并为未来努力实现其在体内的最佳使用奠定了基础。
更新日期:2021-11-24
中文翻译:

负载碳硼烷的治疗诊断纳米颗粒靶向前列腺特异性膜抗原的合成和初步生物学评估
硼中子俘获疗法 (BNCT) 是一种令人鼓舞的癌症治疗方法。前列腺特异性膜抗原 (PSMA) 是一种细胞膜蛋白,在前列腺癌中大量过度表达,可以用放射配体疗法靶向刺激患者的临床反应。原则上,空间靶向中子束与特异性靶向 PSMA 配体一起可以实现前列腺癌靶向 BNCT。因此,我们开发并测试了 PSMA 靶向的聚(丙交酯-共-乙交酯)-嵌段-聚(乙二醇)(PLGA- b -PEG)纳米粒子(NPs),负载有碳硼烷并与放射性金属螯合剂去铁胺 B(DFB)相连用于同时正电子发射断层扫描 (PET) 成像和选择性地将硼输送到前列腺癌。单体PLGA-b -PEG 与 DFB 或 PSMA 配体 ACUPA 共价官能化。不同的纳米颗粒制剂通过相应的未改性和 DFB 或 ACUPA 改性单体以不同的百分比分数进行纳米乳化而产生。纳米粒子被89 Zr有效标记,并进行了体外和体内评估。优化的DFB(25)ACUPA(75) NPs 在PSMA(+) PC3-Pip 细胞中的直接结合和竞争放射性配体结合试验中表现出与PSMA 的强体外结合。[ 89 Zr]DFB(25) NPs 和 [ 89Zr]DFB(25)ACUPA(75) NPs 被注射到具有双侧 PSMA(-) PC3-Flu 和 PSMA(+) PC3-Pip 双异种移植物的小鼠体内。NPs 在 PC3-Pip 肿瘤中的积累是 PC3-Flu 肿瘤的两倍,肿瘤/血液比为 25;然而,没有检测到 ACUPA 配体的实质性影响。此外,观察到碳硼烷从 NP 中快速释放,导致体内向肿瘤的低硼输送。总之,这些数据证明了 PSMA 靶向、负载碳硼烷的 PLGA- b - PEG 纳米颗粒的合成、表征和初步生物学评估,并为未来努力实现其在体内的最佳使用奠定了基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号