当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of Efficient Non-reducing Enzymatic and Chemical Ligation Strategies for Complex Disulfide-Rich Peptides
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2021-11-09 , DOI: 10.1021/acs.bioconjchem.1c00452 Hue N T Tran 1 , Poanna Tran 1 , Jennifer R Deuis 1 , Kirsten L McMahon 1 , Kuok Yap 1 , David J Craik 1 , Irina Vetter 1, 2 , Christina I Schroeder 1, 3
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2021-11-09 , DOI: 10.1021/acs.bioconjchem.1c00452 Hue N T Tran 1 , Poanna Tran 1 , Jennifer R Deuis 1 , Kirsten L McMahon 1 , Kuok Yap 1 , David J Craik 1 , Irina Vetter 1, 2 , Christina I Schroeder 1, 3
Affiliation
|
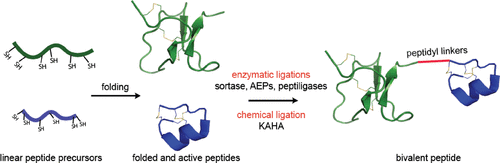
Double-knotted peptides identified in venoms and synthetic bivalent peptide constructs targeting ion channels are emerging tools for the study of ion channel pharmacology and physiology. These highly complex and disulfide-rich peptides contain two individual cystine knots, each comprising six cysteines and three disulfide bonds. Until now, native double-knotted peptides, such as Hi1a and DkTx, have only been isolated from venom or produced recombinantly, whereas engineered double-knotted peptides have successfully been produced through enzymatic ligation using sortase A to form a seamless amide bond at the ligation site between two knotted toxins, and by alkyne/azide click chemistry, joining two peptide knots via a triazole linkage. To further pursue these double-knotted peptides as pharmacological tools or probes for therapeutically relevant ion channels, we sought to identify a robust methodology resulting in a high yield product that lends itself to rapid production and facile mutational studies. In this study, we evaluated the ligation efficiency of enzymatic (sortase A5°, butelase 1, wild-type OaAEP 1, C247A-OaAEP 1, and peptiligase) and mild chemical approaches (α-ketoacid-hydroxylamine, KAHA) for forming a native amide bond linking the toxins while maintaining the native disulfide connectivity of each pre-folded peptide. We used two NaV1.7 inhibitors: PaurTx3, a spider-derived gating modifier peptide, and KIIIA, a small cone snail-derived pore blocker peptide, which have previously been shown to increase affinity and inhibitory potency on hNaV1.7 when ligated together. Correctly folded peptides were successfully ligated in varying yields, without disulfide bond shuffling or reduction, with sortase A5° being the most efficient, resulting in 60% ligation conversion within 15 min. In addition, electrophysiology studies demonstrated that for these two peptides, the amino acid composition of the linker did not affect the activity of the double-knotted peptides. This study demonstrates the powerful application of enzymes in efficiently ligating complex disulfide-rich peptides, paving the way for facile production of double-knotted peptides.
中文翻译:

富含二硫键的复杂肽的有效非还原酶和化学连接策略的评估
在毒液中鉴定的双结肽和针对离子通道的合成二价肽结构是研究离子通道药理学和生理学的新兴工具。这些高度复杂且富含二硫键的肽含有两个单独的胱氨酸结,每个包含六个半胱氨酸和三个二硫键。到目前为止,天然双结肽,如 Hi1a 和 DkTx,仅从毒液中分离或重组生产,而工程化双结肽已通过使用分选酶 A 进行酶连接,在连接处形成无缝酰胺键,成功生产出来两个打结毒素之间的位点,并通过炔/叠氮化物点击化学,通过三唑键连接两个肽结。为了进一步将这些双结肽作为治疗相关离子通道的药理学工具或探针,我们试图找到一种稳健的方法,产生高产量的产品,使其适合快速生产和简便的突变研究。在本研究中,我们评估了酶(分选酶 A5°、丁酶 1、野生型 OaAEP 1、C247A-OaAEP 1 和肽连接酶)和温和化学方法(α-酮酸-羟胺,KAHA)形成天然产物的连接效率。连接毒素的酰胺键,同时保持每个预折叠肽的天然二硫键连接性。我们使用了两种 Na V 1.7 抑制剂:PaurTx3(一种蜘蛛衍生的门控修饰肽)和 KIIIA(一种小锥蜗牛衍生的孔阻断肽),先前已证明它们连接在一起时可增加对 hNa V 1.7 的亲和力和抑制效力。 正确折叠的肽以不同的产量成功连接,没有二硫键改组或还原,分选酶 A5° 是最有效的,在 15 分钟内实现 60% 的连接转化。此外,电生理学研究表明,对于这两种肽,接头的氨基酸组成不会影响双结肽的活性。这项研究证明了酶在有效连接富含二硫键的复杂肽方面的强大应用,为轻松生产双结肽铺平了道路。
更新日期:2021-11-17
中文翻译:

富含二硫键的复杂肽的有效非还原酶和化学连接策略的评估
在毒液中鉴定的双结肽和针对离子通道的合成二价肽结构是研究离子通道药理学和生理学的新兴工具。这些高度复杂且富含二硫键的肽含有两个单独的胱氨酸结,每个包含六个半胱氨酸和三个二硫键。到目前为止,天然双结肽,如 Hi1a 和 DkTx,仅从毒液中分离或重组生产,而工程化双结肽已通过使用分选酶 A 进行酶连接,在连接处形成无缝酰胺键,成功生产出来两个打结毒素之间的位点,并通过炔/叠氮化物点击化学,通过三唑键连接两个肽结。为了进一步将这些双结肽作为治疗相关离子通道的药理学工具或探针,我们试图找到一种稳健的方法,产生高产量的产品,使其适合快速生产和简便的突变研究。在本研究中,我们评估了酶(分选酶 A5°、丁酶 1、野生型 OaAEP 1、C247A-OaAEP 1 和肽连接酶)和温和化学方法(α-酮酸-羟胺,KAHA)形成天然产物的连接效率。连接毒素的酰胺键,同时保持每个预折叠肽的天然二硫键连接性。我们使用了两种 Na V 1.7 抑制剂:PaurTx3(一种蜘蛛衍生的门控修饰肽)和 KIIIA(一种小锥蜗牛衍生的孔阻断肽),先前已证明它们连接在一起时可增加对 hNa V 1.7 的亲和力和抑制效力。 正确折叠的肽以不同的产量成功连接,没有二硫键改组或还原,分选酶 A5° 是最有效的,在 15 分钟内实现 60% 的连接转化。此外,电生理学研究表明,对于这两种肽,接头的氨基酸组成不会影响双结肽的活性。这项研究证明了酶在有效连接富含二硫键的复杂肽方面的强大应用,为轻松生产双结肽铺平了道路。






























 京公网安备 11010802027423号
京公网安备 11010802027423号