Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Precise Delivery of Nanomedicines to M2 Macrophages by Combining “Eat Me/Don’t Eat Me” Signals and Its Anticancer Application
ACS Nano ( IF 15.8 ) Pub Date : 2021-11-09 , DOI: 10.1021/acsnano.1c06707 Yixuan Tang 1, 2 , Zhongjie Tang 2 , Pingrong Li 2 , Kaicheng Tang 2 , Zhongyi Ma 2 , Yantong Wang 2 , Xiaoyou Wang 2 , Chong Li 2
ACS Nano ( IF 15.8 ) Pub Date : 2021-11-09 , DOI: 10.1021/acsnano.1c06707 Yixuan Tang 1, 2 , Zhongjie Tang 2 , Pingrong Li 2 , Kaicheng Tang 2 , Zhongyi Ma 2 , Yantong Wang 2 , Xiaoyou Wang 2 , Chong Li 2
Affiliation
|
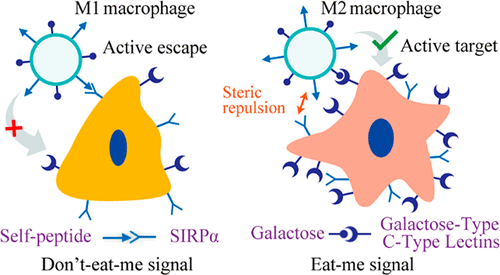
Targeted delivery of nanomedicines to M2 tumor-associated macrophages (TAMs) has been proposed to reduce tumor promotion and enhance the efficacy of anticancer therapy. However, upregulated receptors on M2 TAMs are also expressed on M1 TAMs and other macrophages in normal tissues. Therefore, improving targeting specificity remains a key challenge. Here, we developed a precise M2 TAM-targeted delivery system using “eat-me” and “don’t-eat-me” signals. A CD47-derived self-peptide ligand (don’t-eat-me signal) and galactose ligand (eat-me signal) were introduced on liposomes. Cleavable phospholipid-polyethylene glycol was covered on the surface and could combine with the self-peptide to inhibit macrophage recognition even after immunoglobulin M adsorption and protect galactose from hepatic clearance to prolong the circulation time and promote the accumulation of liposomes in tumors. This detachable polymer can be removed by the redox microenvironment upon transcytosis through the tumor endothelium and re-expose the self-peptide and galactose. The self-peptide highly reduced M1 macrophage phagocytosis, and the galactose ligand enhanced the interaction between the liposomes and M2 macrophages. Thus, the modified liposomes enabled specific recognition of M1/M2 TAMs. In vitro evidence revealed reduced endocytosis of the liposomes by M1 macrophages. Moreover, in vivo studies demonstrated that doxorubicin-loaded liposomes efficiently eliminated M2 TAMs but did not affect M1 TAMs, enhancing the potency of the antitumor therapy. Collectively, our results demonstrate the potential of combining active escape and active targeting for precisely delivering a drug of interest to M2 macrophages and suggest its application in anticancer therapy.
中文翻译:

结合“吃我/不吃我”信号精准递送纳米药物至M2巨噬细胞及其抗癌应用
已经提出将纳米药物靶向递送至 M2 肿瘤相关巨噬细胞 (TAM) 以减少肿瘤促进并增强抗癌治疗的功效。然而,M2 TAM 上调的受体也在正常组织中的 M1 TAM 和其他巨噬细胞上表达。因此,提高靶向特异性仍然是一个关键挑战。在这里,我们使用“吃我”和“不要吃我”信号开发了一个精确的 M2 TAM 靶向传递系统。在脂质体上引入了 CD47 衍生的自身肽配体(不要吃我信号)和半乳糖配体(吃我信号)。可裂解的磷脂-聚乙二醇覆盖在表面,与自身肽结合,抑制巨噬细胞识别免疫球蛋白M后的识别,保护半乳糖不被肝脏清除,从而延长循环时间,促进脂质体在肿瘤中的积累。这种可分离的聚合物可以在通过肿瘤内皮细胞转胞吞作用时被氧化还原微环境去除,并重新暴露自身肽和半乳糖。自身肽大大降低了 M1 巨噬细胞的吞噬作用,半乳糖配体增强了脂质体与 M2 巨噬细胞之间的相互作用。因此,修饰的脂质体能够特异性识别 M1/M2 TAM。这种可分离的聚合物可以在通过肿瘤内皮细胞转胞吞作用时被氧化还原微环境去除,并重新暴露自身肽和半乳糖。自身肽大大降低了 M1 巨噬细胞的吞噬作用,半乳糖配体增强了脂质体与 M2 巨噬细胞之间的相互作用。因此,修饰的脂质体能够特异性识别 M1/M2 TAM。这种可分离的聚合物可以在通过肿瘤内皮细胞转胞吞作用时被氧化还原微环境去除,并重新暴露自身肽和半乳糖。自身肽大大降低了 M1 巨噬细胞的吞噬作用,半乳糖配体增强了脂质体与 M2 巨噬细胞之间的相互作用。因此,修饰的脂质体能够特异性识别 M1/M2 TAM。体外证据显示 M1 巨噬细胞对脂质体的内吞作用减少。此外,体内研究表明,载有阿霉素的脂质体可有效消除 M2 TAM,但不影响 M1 TAM,从而增强抗肿瘤治疗的效力。总的来说,我们的结果证明了将主动逃逸和主动靶向相结合以将感兴趣的药物精确递送至 M2 巨噬细胞的潜力,并表明其在抗癌治疗中的应用。
更新日期:2021-11-23
中文翻译:

结合“吃我/不吃我”信号精准递送纳米药物至M2巨噬细胞及其抗癌应用
已经提出将纳米药物靶向递送至 M2 肿瘤相关巨噬细胞 (TAM) 以减少肿瘤促进并增强抗癌治疗的功效。然而,M2 TAM 上调的受体也在正常组织中的 M1 TAM 和其他巨噬细胞上表达。因此,提高靶向特异性仍然是一个关键挑战。在这里,我们使用“吃我”和“不要吃我”信号开发了一个精确的 M2 TAM 靶向传递系统。在脂质体上引入了 CD47 衍生的自身肽配体(不要吃我信号)和半乳糖配体(吃我信号)。可裂解的磷脂-聚乙二醇覆盖在表面,与自身肽结合,抑制巨噬细胞识别免疫球蛋白M后的识别,保护半乳糖不被肝脏清除,从而延长循环时间,促进脂质体在肿瘤中的积累。这种可分离的聚合物可以在通过肿瘤内皮细胞转胞吞作用时被氧化还原微环境去除,并重新暴露自身肽和半乳糖。自身肽大大降低了 M1 巨噬细胞的吞噬作用,半乳糖配体增强了脂质体与 M2 巨噬细胞之间的相互作用。因此,修饰的脂质体能够特异性识别 M1/M2 TAM。这种可分离的聚合物可以在通过肿瘤内皮细胞转胞吞作用时被氧化还原微环境去除,并重新暴露自身肽和半乳糖。自身肽大大降低了 M1 巨噬细胞的吞噬作用,半乳糖配体增强了脂质体与 M2 巨噬细胞之间的相互作用。因此,修饰的脂质体能够特异性识别 M1/M2 TAM。这种可分离的聚合物可以在通过肿瘤内皮细胞转胞吞作用时被氧化还原微环境去除,并重新暴露自身肽和半乳糖。自身肽大大降低了 M1 巨噬细胞的吞噬作用,半乳糖配体增强了脂质体与 M2 巨噬细胞之间的相互作用。因此,修饰的脂质体能够特异性识别 M1/M2 TAM。体外证据显示 M1 巨噬细胞对脂质体的内吞作用减少。此外,体内研究表明,载有阿霉素的脂质体可有效消除 M2 TAM,但不影响 M1 TAM,从而增强抗肿瘤治疗的效力。总的来说,我们的结果证明了将主动逃逸和主动靶向相结合以将感兴趣的药物精确递送至 M2 巨噬细胞的潜力,并表明其在抗癌治疗中的应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号