当前位置:
X-MOL 学术
›
Drug Metab. Dispos.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Permeabilized Cryopreserved Human Hepatocytes as an Exogenous Metabolic System in a Novel Metabolism-Dependent Cytotoxicity Assay for the Evaluation of Metabolic Activation and Detoxification of Drugs Associated with Drug-Induced Liver Injuries: Results with Acetaminophen, Amiodarone, Cyclophosphamide, Ketoconazole, Nefazodone, and Troglitazone
Drug Metabolism and Disposition ( IF 4.4 ) Pub Date : 2022-02-01 , DOI: 10.1124/dmd.121.000645 Hong Wei 1 , Albert P Li 2
Drug Metabolism and Disposition ( IF 4.4 ) Pub Date : 2022-02-01 , DOI: 10.1124/dmd.121.000645 Hong Wei 1 , Albert P Li 2
Affiliation
|
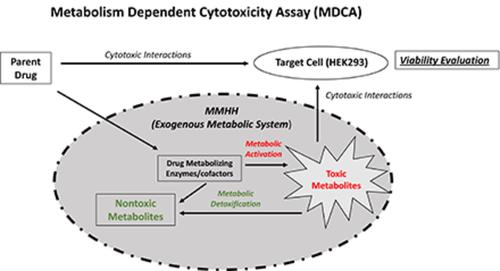
We report here a novel in vitro experimental system, the metabolism-dependent cytotoxicity assay (MDCA), for the definition of the roles of hepatic drug metabolism in toxicity. MDCA employs permeabilized cofactor-supplemented cryopreserved human hepatocytes (MetMax Human Hepatocytes, MMHH), as an exogenous metabolic activating system, and human embryonic kidney 293 (HEK293) cells, a cell line devoid of drug-metabolizing enzyme activity, as target cells for the quantification of drug toxicity. The assay was performed in the presence and absence of cofactors for key drug metabolism pathways known to play key roles in drug toxicity: NADPH/NAD+ for phase 1 oxidation, uridine 5′-diphosphoglucuronic acid (UDPGA) for uridine 5′-diphospho-glucuronosyltransferase (UGT) mediated glucuronidation, 3′-phosphoadenosine-5′-phosphosulfate (PAPS) for cytosolic sulfotransferase (SULT) mediated sulfation, and glutathione (GSH) for glutathione S-transferase (GST) mediated GSH conjugation. Six drugs with clinically significant hepatoxicity, resulting in liver failure or a need for liver transplantation: acetaminophen, amiodarone, cyclophosphamide, ketoconazole, nefazodone, and troglitazone were evaluated. All six drugs exhibited cytotoxicity enhancement by NADPH/NAD+, suggesting metabolic activation via phase 1 oxidation. Attenuation of cytotoxicity by UDPGA was observed for acetaminophen, ketoconazole, and troglitazone, by PAPS for acetaminophen, ketoconazole, and troglitazone, and by GSH for all six drugs. Our results suggest that MDCA can be applied toward the elucidation of metabolic activation and detoxification pathways, providing information that can be applied in drug development to guide structure optimization to reduce toxicity and to aid the assessment of metabolism-based risk factors for drug toxicity. GSH detoxification represents an endpoint for the identification of drugs forming cytotoxic reactive metabolites, a key property of drugs with idiosyncratic hepatotoxicity.
中文翻译:

透化冷冻保存的人肝细胞作为一种新的代谢依赖性细胞毒性试验中的外源代谢系统,用于评估与药物性肝损伤相关的药物的代谢活化和解毒:对乙酰氨基酚、胺碘酮、环磷酰胺、酮康唑、奈法唑酮和曲格列酮的结果
我们在此报告了一种新的体外实验系统,即代谢依赖性细胞毒性试验 (MDCA),用于定义肝脏药物代谢在毒性中的作用。MDCA 使用透化辅因子补充冷冻保存的人肝细胞 (MetMax Human Hepatocytes, MMHH) 作为外源性代谢激活系统,使用人胚肾 293 (HEK293) 细胞(一种缺乏药物代谢酶活性的细胞系)作为靶细胞药物毒性的量化。该测定是在存在和不存在已知在药物毒性中起关键作用的关键药物代谢途径的辅助因子的情况下进行的:NADPH/NAD+ 用于 1 期氧化,尿苷 5'-二磷酸葡萄糖醛酸 (UDPGA) 用于尿苷 5'-二磷酸-葡萄糖醛酸转移酶(UGT) 介导的葡萄糖醛酸化,3'-磷酸腺苷-5'-磷酸硫酸盐 (PAPS) 用于胞质磺基转移酶 (SULT) 介导的硫酸化,谷胱甘肽 (GSH) 用于谷胱甘肽 S-转移酶 (GST) 介导的 GSH 结合。评估了六种具有临床显着肝毒性、导致肝功能衰竭或需要肝移植的药物:对乙酰氨基酚、胺碘酮、环磷酰胺、酮康唑、奈法唑酮和曲格列酮。所有六种药物都表现出 NADPH/NAD+ 的细胞毒性增强作用,表明通过 1 期氧化激活代谢。UDPGA 对对乙酰氨基酚、酮康唑和曲格列酮的细胞毒性减弱,PAPS 对对乙酰氨基酚、酮康唑和曲格列酮的细胞毒性减弱,GSH 对所有六种药物的细胞毒性减弱。我们的研究结果表明,MDCA 可用于阐明代谢激活和解毒途径,提供可用于药物开发的信息,以指导结构优化以降低毒性并帮助评估基于代谢的药物毒性风险因素。GSH 解毒是鉴定形成细胞毒性反应性代谢物的药物的终点,这是具有异质肝毒性药物的关键特性。
更新日期:2022-01-18
中文翻译:

透化冷冻保存的人肝细胞作为一种新的代谢依赖性细胞毒性试验中的外源代谢系统,用于评估与药物性肝损伤相关的药物的代谢活化和解毒:对乙酰氨基酚、胺碘酮、环磷酰胺、酮康唑、奈法唑酮和曲格列酮的结果
我们在此报告了一种新的体外实验系统,即代谢依赖性细胞毒性试验 (MDCA),用于定义肝脏药物代谢在毒性中的作用。MDCA 使用透化辅因子补充冷冻保存的人肝细胞 (MetMax Human Hepatocytes, MMHH) 作为外源性代谢激活系统,使用人胚肾 293 (HEK293) 细胞(一种缺乏药物代谢酶活性的细胞系)作为靶细胞药物毒性的量化。该测定是在存在和不存在已知在药物毒性中起关键作用的关键药物代谢途径的辅助因子的情况下进行的:NADPH/NAD+ 用于 1 期氧化,尿苷 5'-二磷酸葡萄糖醛酸 (UDPGA) 用于尿苷 5'-二磷酸-葡萄糖醛酸转移酶(UGT) 介导的葡萄糖醛酸化,3'-磷酸腺苷-5'-磷酸硫酸盐 (PAPS) 用于胞质磺基转移酶 (SULT) 介导的硫酸化,谷胱甘肽 (GSH) 用于谷胱甘肽 S-转移酶 (GST) 介导的 GSH 结合。评估了六种具有临床显着肝毒性、导致肝功能衰竭或需要肝移植的药物:对乙酰氨基酚、胺碘酮、环磷酰胺、酮康唑、奈法唑酮和曲格列酮。所有六种药物都表现出 NADPH/NAD+ 的细胞毒性增强作用,表明通过 1 期氧化激活代谢。UDPGA 对对乙酰氨基酚、酮康唑和曲格列酮的细胞毒性减弱,PAPS 对对乙酰氨基酚、酮康唑和曲格列酮的细胞毒性减弱,GSH 对所有六种药物的细胞毒性减弱。我们的研究结果表明,MDCA 可用于阐明代谢激活和解毒途径,提供可用于药物开发的信息,以指导结构优化以降低毒性并帮助评估基于代谢的药物毒性风险因素。GSH 解毒是鉴定形成细胞毒性反应性代谢物的药物的终点,这是具有异质肝毒性药物的关键特性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号