当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on the Safety Design of 3-Amino-6-Bromo-1,2,4-Triazine Drying Process Based on Thermal Analysis Technology
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-11-08 , DOI: 10.1021/acs.oprd.1c00293 Zhoushun Zhang 1 , Songsong Li 1 , Lei Chen 1 , Zichao Guo 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-11-08 , DOI: 10.1021/acs.oprd.1c00293 Zhoushun Zhang 1 , Songsong Li 1 , Lei Chen 1 , Zichao Guo 2
Affiliation
|
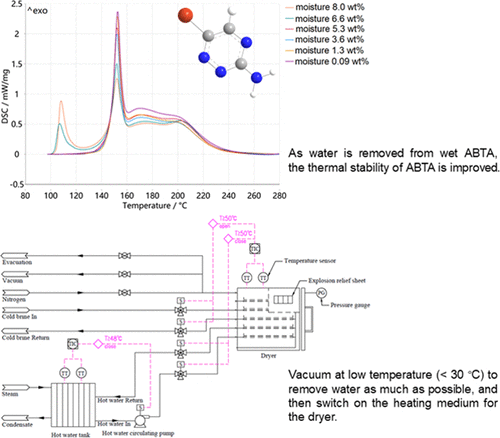
The thermal decomposition behavior of 3-amino-6-bromo-1,2,4-triazine (ABTA) with different moisture contents was studied with a differential scanning calorimeter (DSC). It was found that the thermal stability of the original wet ABTA was worse than that of the qualified dry ABTA. As the drying process proceeded and water was removed from ABTA, the first exothermic shoulder of the original wet ABTA disappeared gradually, and the thermal stability of ABTA improved accordingly. It was verified by the interruption and rescanning method that the decomposition behavior of the qualified dry ABTA followed the N-order reaction, while that of the original wet ABTA had obvious autocatalytic characteristics. The multistep series model was used to fit the thermal decomposition behaviors of the original wet ABTA and the qualified dry ABTA to obtain their decomposition kinetic parameters and predict their heat release behaviors under ideal adiabatic conditions. It was shown that the qualified dry ABTA was safe at 40–50 °C within 48 h, while the original wet ABTA had a great risk of a thermal runaway at 40–50 °C. The Thomas model was used to predict the heat release behavior of the original wet ABTA, which further confirmed the risk of a thermal runaway of the original wet ABTA in the drying process of production. Based on the relevant research results, the safety design requirements and safety operation precautions of the ABTA production drying process were determined.
中文翻译:

基于热分析技术的3-氨基-6-溴-1,2,4-三嗪干燥工艺安全设计研究
用差示扫描量热仪 (DSC) 研究了不同水分含量的 3-氨基-6-溴-1,2,4-三嗪 (ABTA) 的热分解行为。发现原始湿ABTA的热稳定性比合格的干ABTA差。随着干燥过程的进行和ABTA中水分的去除,原始湿ABTA的第一个放热肩逐渐消失,ABTA的热稳定性也相应提高。通过中断再扫描法验证,合格的干ABTA的分解行为遵循N级反应,而原始湿ABTA的分解行为具有明显的自催化特性。采用多步级数模型对原始湿ABTA和合格干ABTA的热分解行为进行拟合,得到其分解动力学参数,预测理想绝热条件下的放热行为。结果表明,合格的干 ABTA 在 40-50°C 下 48 小时内是安全的,而原始湿 ABTA 在 40-50°C 时有很大的热失控风险。采用Thomas模型预测原湿ABTA的放热行为,进一步证实了原湿ABTA在生产干燥过程中发生热失控的风险。根据相关研究成果,确定了ABTA生产干燥过程的安全设计要求和安全操作注意事项。
更新日期:2021-11-19
中文翻译:

基于热分析技术的3-氨基-6-溴-1,2,4-三嗪干燥工艺安全设计研究
用差示扫描量热仪 (DSC) 研究了不同水分含量的 3-氨基-6-溴-1,2,4-三嗪 (ABTA) 的热分解行为。发现原始湿ABTA的热稳定性比合格的干ABTA差。随着干燥过程的进行和ABTA中水分的去除,原始湿ABTA的第一个放热肩逐渐消失,ABTA的热稳定性也相应提高。通过中断再扫描法验证,合格的干ABTA的分解行为遵循N级反应,而原始湿ABTA的分解行为具有明显的自催化特性。采用多步级数模型对原始湿ABTA和合格干ABTA的热分解行为进行拟合,得到其分解动力学参数,预测理想绝热条件下的放热行为。结果表明,合格的干 ABTA 在 40-50°C 下 48 小时内是安全的,而原始湿 ABTA 在 40-50°C 时有很大的热失控风险。采用Thomas模型预测原湿ABTA的放热行为,进一步证实了原湿ABTA在生产干燥过程中发生热失控的风险。根据相关研究成果,确定了ABTA生产干燥过程的安全设计要求和安全操作注意事项。































 京公网安备 11010802027423号
京公网安备 11010802027423号