Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-11-08 , DOI: 10.1016/j.molstruc.2021.131859 Nuthalapati Poojith 1 , Madhuprasad Kigga 2 , J. John Rose 3 , Krishna Murthy Potla 4 , Suneetha Vankayalapati 4, 5 , Sampath Chinnam 6 , Suchetan Parameshwar Adimoole 7 , Renjith Raveendran Pillai 3
|
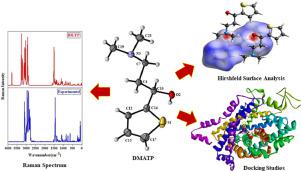
The present study reports structural, spectroscopic signatures, reactivity parameters, and in silico anti-depressant activity of the title compound 3-(dimethylamino)-1-(thiophen-2-yl)propan-1-ol (DMATP). The structure of the DMATP compound has been unambiguously obtained through single crystal X-ray diffraction, vibrational spectroscopy, and thermal (TGA-DTA) analysis. DMATP is a chiral molecule containing a chiral methine carbon with S configuration and because of its chirality the compound crystallizes in the non-centrosymmetric orthorhombic P212121 space group. The thiophene ring and the attached nearly-planar side chain (excluding hydroxyl and one of the methyl group) are oriented to each other making a dihedral angle of 11.2(3)°. The –CH2-CH2 bond at the centre of the side chain has a staggered conformation. In the crystal structure, the molecules are linked via O-H…N hydrogen bonds forming C(6) chains propagating along a-axis resulting in a one dimensional architecture. The intermolecular interactions were analysed qualitatively as well as quantitatively with the aid of 3D-Hirshfeld surface and 2D-fingerprint plot analysis. The optimization of the geometry of the DMATP molecule was performed at DFT/B3LYP/6-311G(d,p) basis set and showed good agreement with the experimental results. The assignment of each vibrational wavenumber was done according to potential energy distribution (PED) analysis. Furthermore, frontier molecular orbital analysis (FMO's), global reactivity parameters, nonlinear optical (NLO) properties, and nonbonding orbital (NBO) analysis were carried out by the same method. Local reactive properties of the title compound were explained by molecular electrostatic potential (MEP), and Fukui functions. The dissociation energies of hydrogen and other single bonds have been also estimated in order to understand the autoxidation mechanism and degradation properties. An anti-depressant study of the DMATP compound was carried out with human serotonin carriers (PDB ID: 5I73) and compiled a binding mechanism with standard anti-depressant drugs such as duloxetine, desvenlafaxine, and atomoxetine.
中文翻译:

3-(二甲氨基)-1-(噻吩-2-基)丙-1-醇的结构、光谱和计算机研究:一种潜在的抗抑郁药
本研究报告了标题化合物 3-(二甲氨基)-1-(噻吩-2-基)丙-1-醇 (DMATP) 的结构、光谱特征、反应性参数和计算机模拟抗抑郁活性。DMATP 化合物的结构已通过单晶 X 射线衍射、振动光谱和热 (TGA-DTA) 分析明确获得。DMATP 是一种手性分子,包含具有 S 构型的手性次甲基碳,并且由于其手性,该化合物在非中心对称斜方晶系P 2 1 2 1 2 1 中结晶空间群。噻吩环和连接的近乎平面的侧链(不包括羟基和甲基之一)彼此取向,形成 11.2(3) °的二面角。侧链中心的–CH 2 -CH 2键具有交错构象。在晶体结构中,分子通过OH…N 氢键形成沿 a 轴传播的 C(6) 链,从而形成一维结构。借助 3D-Hirshfeld 表面和 2D 指纹图分析对分子间相互作用进行定性和定量分析。在 DFT/B3LYP/6-311G(d,p) 基组下对 DMATP 分子的几何结构进行了优化,并显示出与实验结果的良好一致性。根据势能分布(PED)分析完成每个振动波数的分配。此外,前沿分子轨道分析 (FMO)、全局反应性参数、非线性光学 (NLO) 特性和非键轨道 (NBO) 分析通过相同的方法进行。标题化合物的局部反应性质由分子静电势 (MEP) 解释,和福井函数。还估计了氢和其他单键的解离能,以了解自氧化机制和降解特性。使用人血清素载体(PDB ID:5I73)对 DMATP 化合物进行了抗抑郁研究,并编制了与标准抗抑郁药物如度洛西汀、去甲文拉法辛和托莫西汀的结合机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号