Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-11-05 , DOI: 10.1016/j.molstruc.2021.131862 Xingwei Xu 1 , Liping Hu 1 , Mengmeng Fan 1 , Ziwei Hu 1 , Qiming Li 1 , Huan He 1 , Baohui Qi 1
|
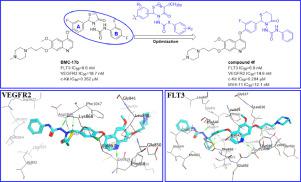
Blockage of multiple oncoprotein or pathway is a more effective approach in current cancer therapy. In this investigation, structural modification was performed on a lead compound, N1-(2,4-difluorophenyl)-N3-(2-(3-fluoro-4-((6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)quinolin-4-yl)oxy)phenyl)-4-oxothiazolidin-3-yl)urea (BMC-17b), which was obtained in our previous study and showed potent inhibitory activities against FLT3 and VEGFR2. Nine novel derivatives were synthesized, and structure-activity relationships (SARs) analysis based on the biological evaluation and docking study led to the discovery of several more potent FLT3/VEGFR2 dual inhibitors. Among them, 1-(2-(3-fluoro-4-((6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)quinolin-4-yl)oxy)phenyl)-4-oxo-1,3-thiazinan-3-yl)-3-phenylurea (4f) possessed significantly inhibitory activities against FLT3, VEGFR2 and FLT3-driven AML MV4-11 cells with IC50 values of 6.9 nM, 14.6 nM and 12.1 nM, respectively. Furthermore, compound 4f exhibited over 41-fold selectivity toward FLT3 relative to c-Kit, which might reduce myelosuppression toxicity.
中文翻译:

鉴定 1,3-thiazinan-4-one 尿素衍生物作为治疗急性髓系白血病的强效 FLT3/VEGFR2 双抑制剂
阻断多种癌蛋白或通路是目前癌症治疗中更有效的方法。在这项研究中,对先导化合物N 1 -(2,4-二氟苯基)- N 3 -(2-(3-fluoro-4-((6-甲氧基-7-(3-(4 -甲基哌嗪-1-基)丙氧基)喹啉-4-基)氧基)苯基)-4-氧噻唑啉-3-基)脲 ( BMC-17b),这是在我们之前的研究中获得的,并显示出对 FLT3 和 VEGFR2 的有效抑制活性。合成了九种新型衍生物,基于生物学评价和对接研究的构效关系 (SAR) 分析导致发现了几种更有效的 FLT3/VEGFR2 双抑制剂。其中,1-(2-(3-氟-4-((6-甲氧基-7-(3-(4-甲基哌嗪-1-基)丙氧基)喹啉-4-基)氧基)苯基)-4- oxo-1,3-thiazinan-3-yl)-3-phenylurea ( 4f ) 对 FLT3、VEGFR2 和 FLT3 驱动的 AML MV4-11 细胞具有显着的抑制活性,IC 50值为 6.9 nM、14.6 nM 和 12.1 nM,分别。此外,化合物4f 相对于 c-Kit,对 FLT3 的选择性超过 41 倍,这可能会降低骨髓抑制毒性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号