当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ni-Catalyzed Formal Cross-Electrophile Coupling of Alcohols with Aryl Halides
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-11-05 , DOI: 10.1021/acscatal.1c04239 Quan Lin 1 , Guobin Ma 2 , Hegui Gong 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-11-05 , DOI: 10.1021/acscatal.1c04239 Quan Lin 1 , Guobin Ma 2 , Hegui Gong 2
Affiliation
|
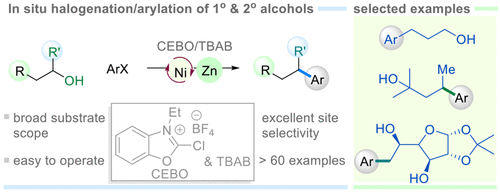
Direct coupling of unactivated alcohols remains a challenge in current synthetic chemistry. We herein demonstrate a strategy building upon in situ halogenation/reductive coupling of alcohols with aryl halides to forge Csp2–Csp3 bonds. The combination of 2-chloro-3-ethylbenzo[d]oxazol-3-ium salt (CEBO) and TBAB as the mild bromination reagents enables rapid transformation of a wide range of alcohols to their bromide counterparts within one to 5 min in CH3CN and DMF, which is compatible with the Ni-catalyzed cross-electrophile coupling conditions in the presence of a chemical reductant. The present method is suitable for arylation of a myriad of structurally complex alcohols with no need for prepreparation of alkyl halides. More importantly, the mild and kinetically rapid bromination process has shown good selectivity in the bromination/arylation of symmetric diols and less sterically hindered hydroxyl groups in polyols, thus offering promise for selective functionalization of diols and polyols without laborious protecting/deprotecting operations. The practicality of this work is also evident in the arylation of a number of carbohydrates, drug compounds, and naturally occurring alcohols.
中文翻译:

Ni催化醇与芳基卤化物的正式交叉电子偶联
未活化醇的直接偶联仍然是当前合成化学中的一个挑战。我们在此展示了一种建立在醇与芳基卤化物的原位卤化/还原偶联以形成 Csp 2 -Csp 3键的策略。2-氯-3-乙基苯并[ d ]恶唑-3-鎓盐 (CEBO) 和 TBAB 作为温和溴化试剂的组合能够在 CH 3 中在一到 5 分钟内将范围广泛的醇快速转化为其溴化物对应物CN 和 DMF,在化学还原剂存在下与 Ni 催化的交叉亲电偶联条件相容。本方法适用于无数结构复杂的醇的芳基化,而无需制备卤代烷。更重要的是,温和且动力学快速的溴化过程在对称二醇的溴化/芳基化和多元醇中较少的空间位阻羟基中显示出良好的选择性,从而为二醇和多元醇的选择性官能化提供了希望,而无需费力的保护/脱保护操作。这项工作的实用性在许多碳水化合物、药物化合物和天然醇的芳基化中也很明显。
更新日期:2021-11-19
中文翻译:

Ni催化醇与芳基卤化物的正式交叉电子偶联
未活化醇的直接偶联仍然是当前合成化学中的一个挑战。我们在此展示了一种建立在醇与芳基卤化物的原位卤化/还原偶联以形成 Csp 2 -Csp 3键的策略。2-氯-3-乙基苯并[ d ]恶唑-3-鎓盐 (CEBO) 和 TBAB 作为温和溴化试剂的组合能够在 CH 3 中在一到 5 分钟内将范围广泛的醇快速转化为其溴化物对应物CN 和 DMF,在化学还原剂存在下与 Ni 催化的交叉亲电偶联条件相容。本方法适用于无数结构复杂的醇的芳基化,而无需制备卤代烷。更重要的是,温和且动力学快速的溴化过程在对称二醇的溴化/芳基化和多元醇中较少的空间位阻羟基中显示出良好的选择性,从而为二醇和多元醇的选择性官能化提供了希望,而无需费力的保护/脱保护操作。这项工作的实用性在许多碳水化合物、药物化合物和天然醇的芳基化中也很明显。






































 京公网安备 11010802027423号
京公网安备 11010802027423号