当前位置:
X-MOL 学术
›
J. Food Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular cloning and characterization of a novel xylanase from Microbacterium imperiale YD-01
Journal of Food Biochemistry ( IF 3.5 ) Pub Date : 2021-11-03 , DOI: 10.1111/jfbc.13988 Keqin Tang 1, 2 , Yin Cui 2 , Jingyi Xiao 3 , Mengyao Ding 2 , Hongjun Chao 2 , Jing Wu 2 , Zhenggang Han 2 , Jun Liu 2 , Xin Li 2 , Dazhong Yan 1, 2
Journal of Food Biochemistry ( IF 3.5 ) Pub Date : 2021-11-03 , DOI: 10.1111/jfbc.13988 Keqin Tang 1, 2 , Yin Cui 2 , Jingyi Xiao 3 , Mengyao Ding 2 , Hongjun Chao 2 , Jing Wu 2 , Zhenggang Han 2 , Jun Liu 2 , Xin Li 2 , Dazhong Yan 1, 2
Affiliation
|
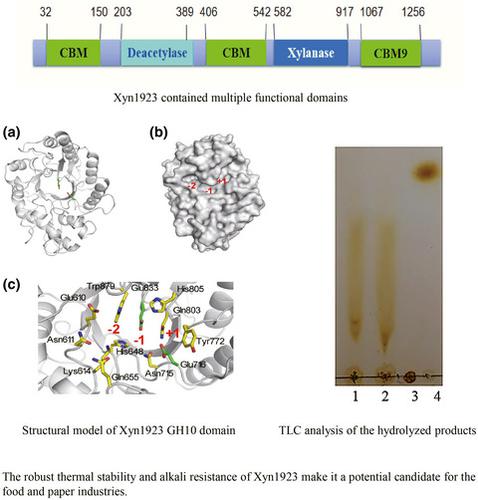
Xylaneses are very common xylanolytic enzymes, which are widely used in food, papermaking, and other industries. In this study, a xylanase-encoding gene xyn1923, which encodes a protein of 1352 amino acids, was identified through the whole genome analysis of Microbacterium imperiale YD-01. Bioinformatics analysis showed that Xyn1923 only had maximum similarity of 37% with the reported xylanase from Alkalihalobacillus halodurans C-125, indicating that Xyn1923 was a novel xylanase. The enzymatic properties of Xyn1923 were systematically analyzed after purification. The results showed that the specific activity of the enzyme was 10.582 ± 0.413 U/mg, while the optimum pH and temperature of the enzyme were 7.0 and 70°C, respectively. The enzyme is stable in the pH range of 6.0–9.0, and the enzyme activity could maintain more than 85% of the original activity after 16 hr incubation at pH 9.0. The enzyme activity is relatively stable in the range of 30–60°C, and its enzyme activity could maintain more than 89% of the original activity after treatment at 60°C for 30 min. Low concentrations (≤1 mM) of Co2+, Ba2+, Fe2+, and Fe3+ metal ions exerted a stimulatory effect on the activity of Xyn1923. And in contrast, high concentrations (≥2 mM) of the above metal ions inhibit the activity of Xyn1923. Mg2+, Ag+, Cu2+, Ca2+, Mn2+, and Pb2+ ions showed a negative effect on the activity of Xyn1923. Enzyme kinetic studies showed that Km and Vmax values for xylan were 7.842 ± 0.538 mg/ml and 15.208 ± 0.822 U/mg, respectively. Xyn1923 was found to be a weakly alkaline thermophilic xylanase through an enzymatic property analysis.
中文翻译:

一种来自帝王微杆菌 YD-01 的新型木聚糖酶的分子克隆和表征
木聚糖酶是非常常见的木聚糖分解酶,广泛应用于食品、造纸等行业。本研究通过对Microbacterium Imperiale YD-01的全基因组分析,鉴定出一个编码1352个氨基酸的蛋白质的木聚糖酶编码基因xyn1923 。生物信息学分析表明,Xyn1923 与已报道的来自嗜盐碱盐杆菌的木聚糖酶的最大相似性仅为 37% 。C-125,表明Xyn1923是一种新型木聚糖酶。纯化后对Xyn1923的酶学性质进行了系统分析。结果表明,酶的比活性为10.582±0.413 U/mg,酶的最适pH和温度分别为7.0和70℃。该酶在pH 6.0-9.0范围内稳定,在pH 9.0孵育16小时后,酶活性可维持在原活性的85%以上。酶活力在30~60℃范围内较为稳定,60℃处理30 min后酶活力可维持在原有活力的89%以上。低浓度 (≤1 mM) Co 2+、Ba 2+、Fe 2+和 Fe 3+金属离子对 Xyn1923 的活性有刺激作用。相比之下,高浓度(≥2 mM)的上述金属离子会抑制 Xyn1923 的活性。Mg 2+、Ag +、Cu 2+、Ca 2+、Mn 2+和Pb 2+离子对Xyn1923的活性有负面影响。酶动力学研究表明,木聚糖的K m和V最大值分别为 7.842 ± 0.538 mg/ml 和 15.208 ± 0.822 U/mg。通过酶学性质分析发现 Xyn1923 是一种弱碱性嗜热木聚糖酶。
更新日期:2021-12-09
中文翻译:

一种来自帝王微杆菌 YD-01 的新型木聚糖酶的分子克隆和表征
木聚糖酶是非常常见的木聚糖分解酶,广泛应用于食品、造纸等行业。本研究通过对Microbacterium Imperiale YD-01的全基因组分析,鉴定出一个编码1352个氨基酸的蛋白质的木聚糖酶编码基因xyn1923 。生物信息学分析表明,Xyn1923 与已报道的来自嗜盐碱盐杆菌的木聚糖酶的最大相似性仅为 37% 。C-125,表明Xyn1923是一种新型木聚糖酶。纯化后对Xyn1923的酶学性质进行了系统分析。结果表明,酶的比活性为10.582±0.413 U/mg,酶的最适pH和温度分别为7.0和70℃。该酶在pH 6.0-9.0范围内稳定,在pH 9.0孵育16小时后,酶活性可维持在原活性的85%以上。酶活力在30~60℃范围内较为稳定,60℃处理30 min后酶活力可维持在原有活力的89%以上。低浓度 (≤1 mM) Co 2+、Ba 2+、Fe 2+和 Fe 3+金属离子对 Xyn1923 的活性有刺激作用。相比之下,高浓度(≥2 mM)的上述金属离子会抑制 Xyn1923 的活性。Mg 2+、Ag +、Cu 2+、Ca 2+、Mn 2+和Pb 2+离子对Xyn1923的活性有负面影响。酶动力学研究表明,木聚糖的K m和V最大值分别为 7.842 ± 0.538 mg/ml 和 15.208 ± 0.822 U/mg。通过酶学性质分析发现 Xyn1923 是一种弱碱性嗜热木聚糖酶。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号